Alpha Beta And Gamma Rays Comparison
Therefore they have a mass of approximately where so the mass of an alpha particle is 66410 27 kg 373 gevc 2. Alpha rays vs beta rays vs gamma rays compare alpha particles beta particles and gamma rays table an unstable atomic nuclei loss its energy by emitting radiations such as alpha rays beta rays and gamma rays by a process called radioactive decay.
 Radioactive Decay Lesson 0718 Tqa Explorer
Radioactive Decay Lesson 0718 Tqa Explorer
data.allenai.org
Beta radiation is the producer of fast moving electrons and can penetrate further in comparison to the alpha particles.
Alpha beta and gamma rays comparison. Radiation is all around us. Comparison of alpha particles beta particles and gamma rays. Alpha particles are he 4 nuclei and beta is either electrons or positrons.
Alpha and beta particles gamma and x rays. Which is most dangerous. Well that all depends on the situation and the two properties that this.
Gamma radiation is the deadliest of the three. From radio waves transmitting information to the visible spectrum that gives our world color. But what about the more dangerous forms of radiation.
During radioactivity particles like alpha beta gamma rays are emitted by an atom due to unstable atom trying to gain stability. Alpha radiation can be described as the producer of high energy and fast moving helium particles. Alpha and beta radiation are stream of particles consisting mass.
Alpha beta and gamma radiation all differ on the kind of radioactive particle that is emitted. In gamma radiaton a gamma particle that has an atomic mass value of 0 and a atomic number of 0 is released. Hence the atoms eventually decay by emitting a particle that transforms when they are unstable and transforms the nucleus into a lower energy state.
What is the difference between alpha beta and gamma radiation. Electrons and positrons which make up beta particles are antiparticles of each other. A visual comparison of the properties of alpha beta and gamma radiation using a simple model.
Alpha particles are made of four nucleons. Gamma radiations are high energy radiations that are in the form of electromagnetic waves and these radiations do not give off any particle like alpha and gamma. Heck even talking produces radiation in the form of sound.
What are alpha particles beta particles and gamma rays. Alpha beta and gamma are forms of ionizing radiation and comes from the nuclei of atoms and is an intrinsic part of the environment around us. A substance with such an unstable nucleus is called the radioactive substance.
These photons are energy packets that transport energy from one place to another as a gamma ray. While most atoms remain stable some will disintegrate and transforms them. However in order to understand the behavior of gamma rays and to compare them with alpha and beta particles a hypothetical particle called photon is introduced.
Gamma radiation is an electromagnetic radiation and consists of high energy quanta. Mass of alpha beta and gamma radiation. Therefore they are called gamma particles.
In beta radiation a beta particle with 0 atomic mass and 1 charge an electron is released.
 Physicscentral
Physicscentral
www.physicscentral.com
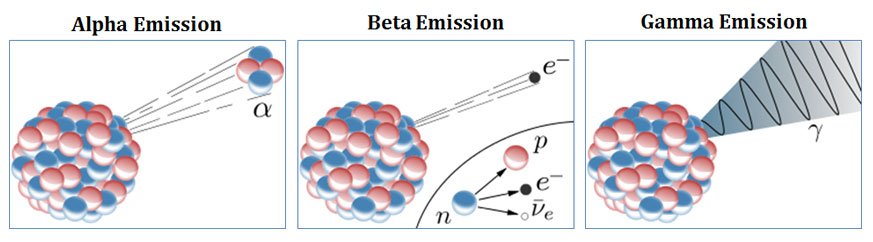 Alpha Rays Vs Beta Rays Vs Gamma Rays Compare Easybiologyclass
Alpha Rays Vs Beta Rays Vs Gamma Rays Compare Easybiologyclass
www.easybiologyclass.com
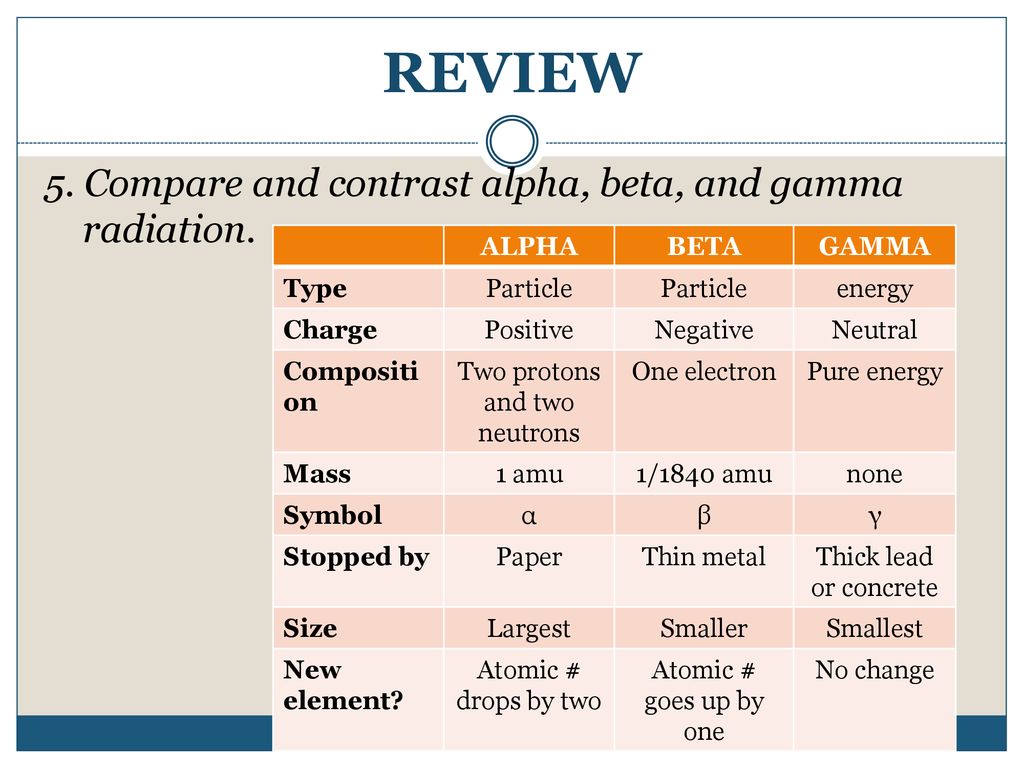 Radioactivity Review Ppt Download
Radioactivity Review Ppt Download
slideplayer.com
Radioactivity And Alpha Beta Gamma Radiations And X Rays
www.oasisllc.com
 Gamma Ray An Overview Sciencedirect Topics
Gamma Ray An Overview Sciencedirect Topics
www.sciencedirect.com
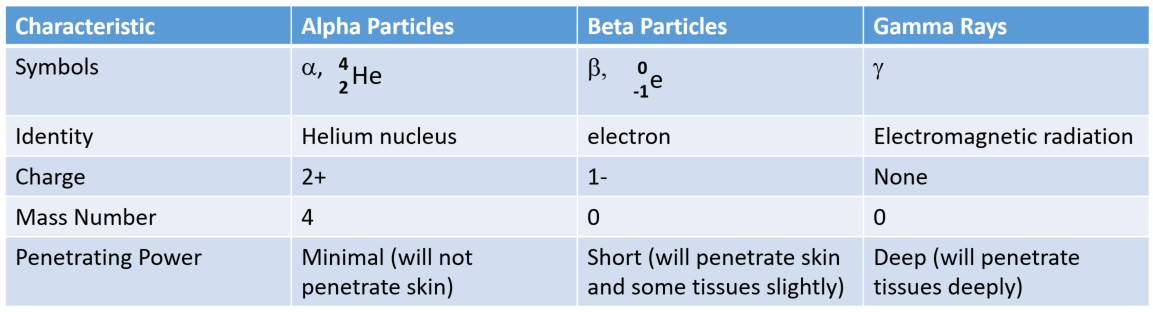 Ch103 Chapter 3 Radioactivity And Nuclear Chemistry Chemistry
Ch103 Chapter 3 Radioactivity And Nuclear Chemistry Chemistry
wou.edu
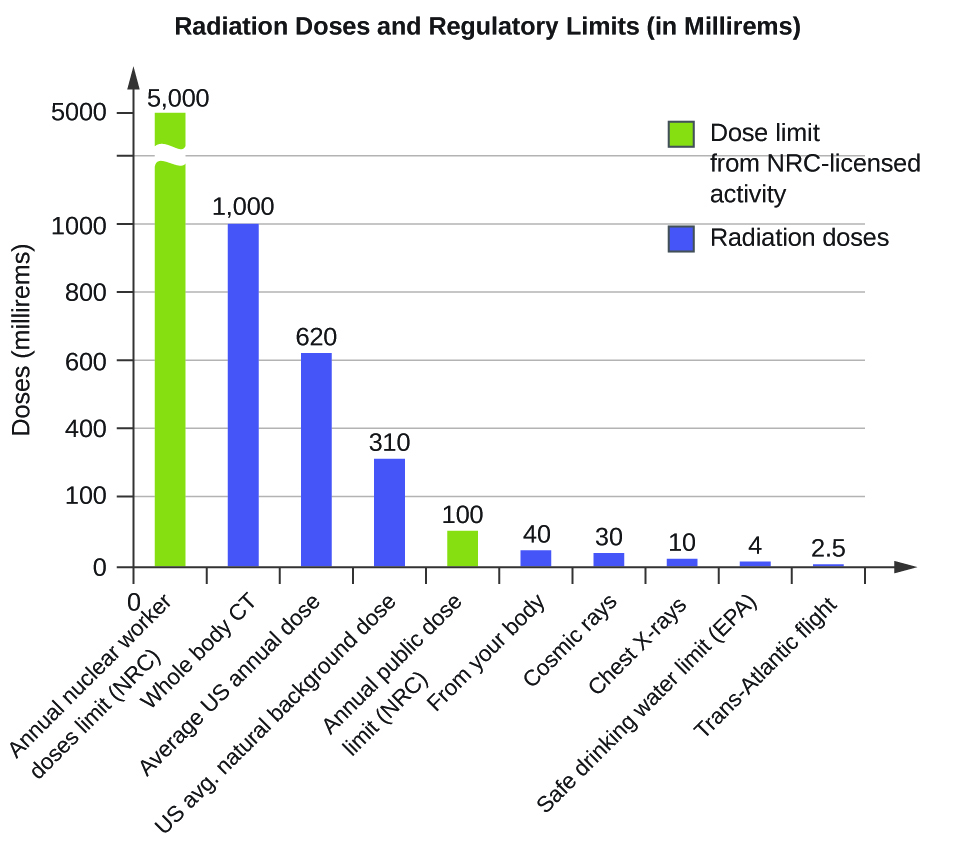 21 6 Biological Effects Of Radiation Chemistry
21 6 Biological Effects Of Radiation Chemistry
opentextbc.ca
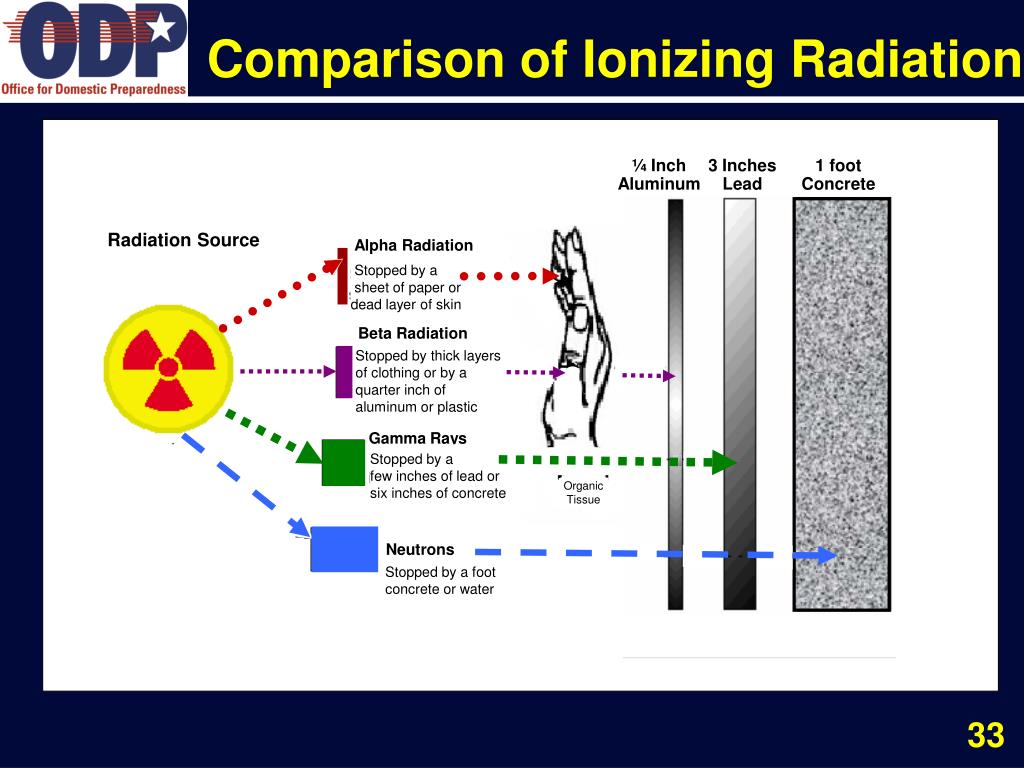 Ppt Module 1 Introduction To Radiation Powerpoint Presentation
Ppt Module 1 Introduction To Radiation Powerpoint Presentation
www.slideserve.com
Difference Between Radiation And Emission Definition Different
pediaa.com
0 Response to "Alpha Beta And Gamma Rays Comparison"
Post a Comment