Alpha Beta And Gamma Radiation Power
These particles have the minimum penetration power and highest ionization power. The emission of particles is also called the emission of radiationthe radiation is emitted from the nucleus of an atom converting protons or neutrons of the nucleus into different particles.
 Alpha Beta And Gamma Rays Hindi Youtube
Alpha Beta And Gamma Rays Hindi Youtube
www.youtube.com
Alpha beta gamma lesson contents physical properties of a b and g penetrating power of a b and g n v z graphs decay laws alpha radiation alpha particles contain two.

Alpha beta and gamma radiation power. The number of cars entering it since the start of the day count. For the moment well say that alpha and beta radiation consist of tiny particles much smaller than an atom. Just as gamma radiation is the excess energy of an excited nucleus after losing an alpha particle x ray radiation is the result of excited electrons.
The number of cars entering it per minute count rate. Their kinetic energy is sufficient to ionize matter. Alpha beta and gamma radiation are types of ionizing radiation.
Alpha rays are the positively charged particles. This decay occurs through emission of different particles. Gamma rays have tremendous penetration power and require several inches of dense material like lead to shield them.
X rays are identical to gamma rays although they originate from a different source. Comparison distinguish difference between. Alphabeta and gamma radiation 1 alphabeta and gamma radiation 2 warm up 1 which of the following is more important for finding out the rush hour of the tunnel.
Lets discuss the properties of beta alpha and gamma one by one. Alpha particle is highly active and energetic helium atom that contains two neutrons and protons. 3 warm up 2 below is the time record of the total.
Therefore they have a mass of approximately where so the mass of an alpha particle is 66410 27 kg 373 gevc 2. Mass of alpha beta and gamma radiation. Main difference alpha vs beta vs gamma particles.
Alpha beta and gamma are the first three letters of the greek alphabet. Alpha particles are made of four nucleons. Gamma rays are energy that has no mass or charge.
Gamma rays are not particles but a high energy form of electromagnetic radiation like x rays except more powerful. They move incredibly fast perhaps thousands of kilometres per second. Radioactivity is a process of decay of chemical elements with time.
Gamma radiation is a sort of invisible very high energy light. Electrons and positrons which make up beta particles are antiparticles of each other. High speed electrons are able to knock off electrons from a material usually a metal.
 Beta Particle Wikipedia
Beta Particle Wikipedia
en.wikipedia.org
Properties Ionising Radiation Alpha Beta Gamma Ionizing
www.docbrown.info

www.tes.com
 Alpha Rays Vs Beta Rays Vs Gamma Rays Compare Easybiologyclass
Alpha Rays Vs Beta Rays Vs Gamma Rays Compare Easybiologyclass
www.easybiologyclass.com
Radioactivity Experiments To Determine The Penetrating Power And
www.markedbyteachers.com
 A Guide To The Different Types Of Radiation Compound Interest
A Guide To The Different Types Of Radiation Compound Interest
www.compoundchem.com
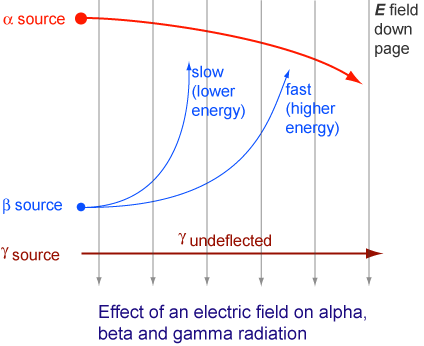 The Sun Energy From Nuclei
The Sun Energy From Nuclei
www.atnf.csiro.au
 Gcse Science Physics Properties Of Radiation Powerpoint And
Gcse Science Physics Properties Of Radiation Powerpoint And
www.tes.com
 Penetration Power Of Ionizing Rays Royalty Free Vector Image
Penetration Power Of Ionizing Rays Royalty Free Vector Image
www.vectorstock.com
0 Response to "Alpha Beta And Gamma Radiation Power"
Post a Comment