Alpha Beta And Gamma Radiation All Show Different Levels Of Penetration Because
The emission of particles is also called the emission of radiationthe radiation is emitted from the nucleus of an atom converting protons or neutrons of the nucleus into different particles. They all have the same mass they all have the same charge they all can be emitted from the nucleus of an atom they all have the same energy alpha beta and gamma radiation all show different levels of penetration because.
Properties Ionising Radiation Alpha Beta Gamma Ionizing
www.docbrown.info
The alpha and beta radiation consist of actual matter that shoots off the atom while gamma rays are electromagnetic waves.
Alpha beta and gamma radiation all show different levels of penetration because. Gamma rays but for alpha and beta rays it is sometimes said. Beta particles are electrons with very high speed. All three kinds of radiation are potentially hazardous to living tissue but some more than others as will be explained later on.
Main difference alpha vs beta vs gamma particles. The lack of charge eliminates coulomb interactions and allows gamma rays to be much more penetrating. Mass of alpha beta and gamma radiation.
This decay occurs through emission of different particles. Heck even talking produces radiation in the form of sound. 3 gamma ray interactions with matter are entirely different from that of charged particles.
Gamma beta and alpha radiation are all alike because. Penetration of gamma rays. Of radioactive atoms these are alpha beta and gamma radiation.
Radioactivity is a process of decay of chemical elements with time. Electrons and positrons which make up beta particles are antiparticles of each other. Beta particles and alpha particles.
When atoms decay they emit three types of radiation alpha beta and gamma. The energies of each type of radiation varies depending on the source therefore every alpha source is not exactly the same and this variation also applies to beta and gamma. What makes these ionizing forms of radiation so different from microwaves for example which we encounter in our lives on a daily basis.
It cannot be said that a particular thickness of a material can absorb all gamma radiation. Therefore it is always said. A 38 96 mm thick piece of plastic is required to stop all the sr 90 betas.
Not so when you switch over to the sr 90 source. Alpha and beta particles gamma and x rays. But what about the more dangerous forms of radiation.
Gamma rays are the most penetrating of the radiations. Please help me i beg you i really need help. Alpha beta and gamma radiation have very different properties in penetrating through materials and being absorbed by them.
Beta particles can be stopped by a few millimetres of aluminium. They can be stopped by a thin metal plate with a thickness of 1 cm. Alpha radiation consists of alpha particlesan alpha particle is identical to the nucleus of a helium atom which.
Alpha particles are made of four nucleons. The other radiations beta and alpha have particle charecter. Therefore they have a mass of approximately where so the mass of an alpha particle is 66410 27 kg 373 gevc 2.
Gamma rays are highly energetic waves and are poor at ionising other atoms or molecules.
 Basic Modes Of Radioactive Decay Intechopen
Basic Modes Of Radioactive Decay Intechopen
www.intechopen.com
Particle Physics Page 2
www.antonine-education.co.uk
 Gcse Physics Alpha Beta And Gamma Radiation 33 Youtube
Gcse Physics Alpha Beta And Gamma Radiation 33 Youtube
www.youtube.com
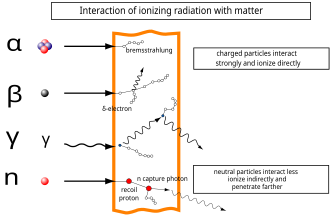 Ionizing Radiation Wikipedia
Ionizing Radiation Wikipedia
en.wikipedia.org
 Basic Modes Of Radioactive Decay Intechopen
Basic Modes Of Radioactive Decay Intechopen
www.intechopen.com
Penetration Of Alpha Beta And Gamma Rays Pass My Exams Easy
www.passmyexams.co.uk
Radioactive Transitions
www.sprawls.org
I am very much pleased with the contents you have mentioned. I wanted to thank you for this great article. Penetration Test
ReplyDelete