Alpha Beta And Gamma Radiation Mass
A beta particle aka beta radiation is a high speed electron or positron. Gamma radiation unlike alpha or beta does not consist of any particles instead consisting of a photon of energy being emitted from an unstable nucleus.
 Buying A Geiger Counter Page 2
Buying A Geiger Counter Page 2
www.imagesco.com
Electrons and positrons which make up beta particles are antiparticles of each other.
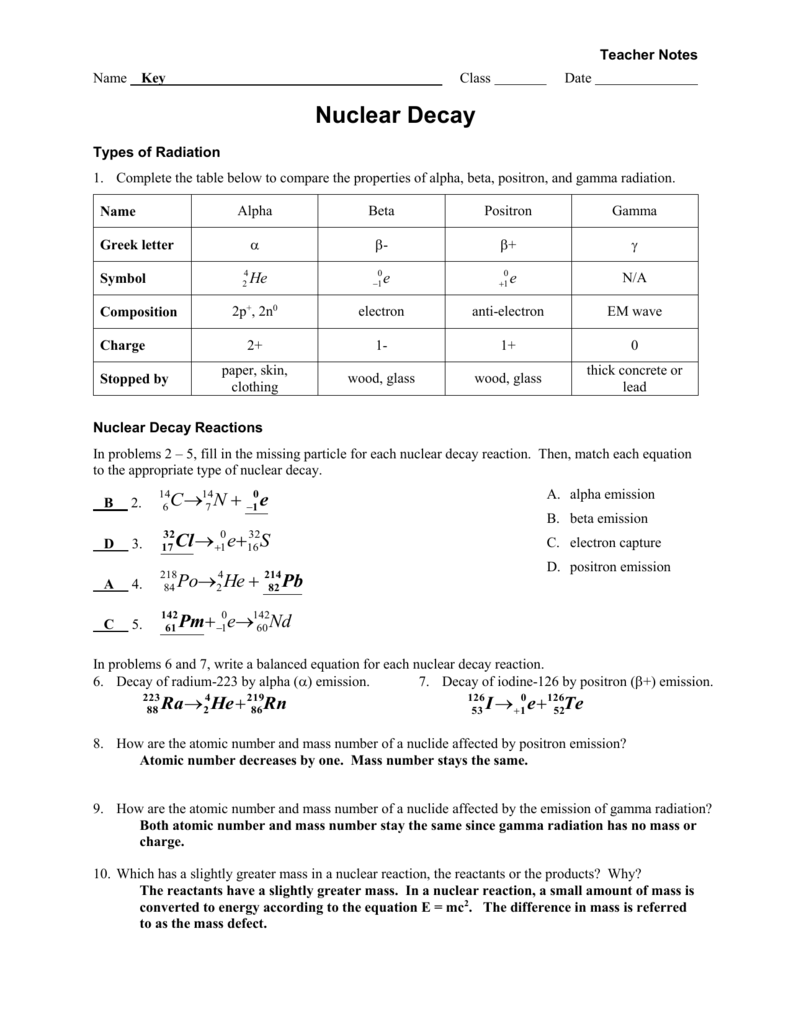
Alpha beta and gamma radiation mass. This decay occurs through emission of different particles. Main difference alpha vs beta vs gamma particles. Gamma rays are a high frequency form of electromagnetic radiation so they travel at the speed of light.
During radioactivity particles like alpha beta gamma rays are emitted by an atom due to unstable atom trying to gain stability. Express the changes in the atomic number and mass number of a radioactive nuclei when an alpha beta or gamma particle is emitted. Therefore they have a mass of approximately where so the mass of an alpha particle is 66410 27 kg 373 gevc 2.
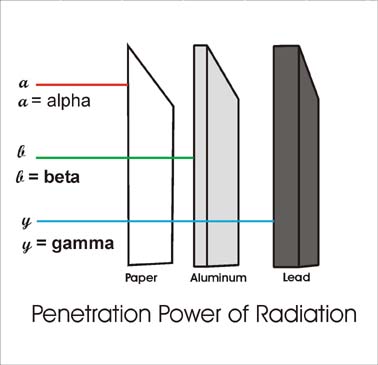
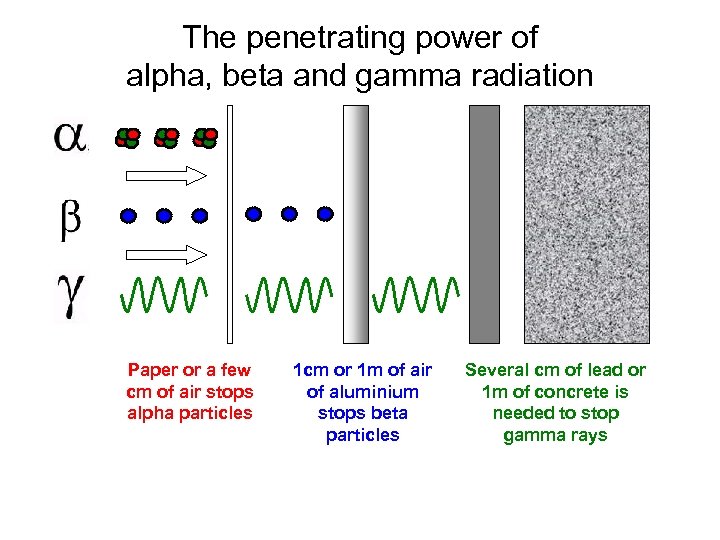
Having no mass or charge gamma radiation can travel much farther through air than alpha or beta losing on average half its energy for every 500 feet. Mass of alpha beta and gamma radiation. The alpha particle basically has the mass of a helium atom since it is a helium nucleus.
In gamma radiaton a gamma particle that has an atomic mass value of 0 and a atomic number of 0 is released. That is they decompose by emitting particles and in doing so become a different nucleus. Alpha or beta emission can leave a nucleus in a higher energy excited state and the energy released as a result of these processes is done in the form of gamma rays.
In beta radiation a beta particle with 0 atomic mass and 1 charge an electron is released. Many nuclei are radioactive. Alpha beta and gamma radiation all differ on the kind of radioactive particle that is emitted.
Alpha particles are made of four nucleons. Gamma radiation is the deadliest of the three. However the nucleus can also end up in a higher energy state after colliding with another nucleus or being struck by a neutron.
Their mass is 12000 amu. When a nucleus ejects an alpha or beta particle it is left in an excited or higher energy state and it can fall to a lower energy state by releasing a gamma ray photon. Write nuclear equations for alpha and beta decay reactions.
The emission of particles is also called the emission of radiationthe radiation is emitted from the nucleus of an atom converting protons or neutrons of the nucleus into different particles. Radioactivity is a process of decay of chemical elements with time. A gamma ray aka gamma radiation i.
Hence the atoms eventually decay by emitting a particle that transforms when they are unstable and transforms the nucleus into a lower energy state. An alpha particle aka alpha radiation is basically a helium nucleus so 2 ea protons neutrons so 4 amu. Emission of gamma rays often follows emission of alpha or beta particles.
The beta particle has the mass of an electron a beta particle is either an electron or an anti electron.
 Worksheet Radioactive Decay Fission Fusion Key
Worksheet Radioactive Decay Fission Fusion Key
studylib.net
 Edexcel Igcse Certificate In Physics 7 1
Edexcel Igcse Certificate In Physics 7 1
present5.com
Radioactive Decay Storyboard Par Phitran
www.storyboardthat.com
 Radiation Basics Radiation Protection Us Epa
Radiation Basics Radiation Protection Us Epa
www.epa.gov
Chapter 21 Section 1
wps.prenhall.com
 Write The Composition Of Positive Alpha Beta Gamma Rays
Write The Composition Of Positive Alpha Beta Gamma Rays
www.scholr.com
Properties Ionising Radiation Alpha Beta Gamma Ionizing
www.docbrown.info
 Alpha Beta And Gamma Rays Properties Radioactive Displacement Law
Alpha Beta And Gamma Rays Properties Radioactive Displacement Law
www.brainkart.com
0 Response to "Alpha Beta And Gamma Radiation Mass"
Post a Comment