Imrt Osmotic Pressure
P is not equal to 314159 in this situation. We calculate the osmotic pressure pi using the following equation.
 Biochem Sapling 3 Review 1 2 3 2 And Lecture Review Notsaem
Biochem Sapling 3 Review 1 2 3 2 And Lecture Review Notsaem
notsaem.wordpress.com
It is also defined as the measure of the tendency of a solution to take in pure solvent by osmosis.

Imrt osmotic pressure. If the solution is hypertonic to the cytoplasm of the red blood cells the cells will shrink through a process called crenationif the solution is hypotonic with respect to the osmotic pressure of the cytoplasm water will rush into the cells to try to reach equilibrium. I don39t know how to do this. Since this is a polymer the value of i is one.
P imrt note how it resembles the pv nrt form of the ideal gas law where p is the osmotic pressure in atm i van t hoff factor of the solute m molar concentration in moll r universal gas constant 008206 latmmolk t absolute temperature in k. I had imagined turgor would get more hits because it is one word but it seems osmotic pressure is the correct term. Based on temperature of the solution this osmolarity will produce a pressure across a membrane called osmotic pressure pi.
The osmosis equation is. M is the molar concentration of dissolved species units of moll. A quick google test gives 100000 results for turgor and 175000 for osmotic pressure.
Osmotic pressure is expressed by the formula. We need to know the molar concentration of dissolved species in order to calculate the osmotic pressure of an aqueous solution. The formula is pi imrt where t is in kelvin.
In lieu of this information i suggest this page be moved to osmotic pressure with appropriate redirects. The answer 359 atm. Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
P stands for the osmotic pressure and is usually expressed in the pressure unit of atmospheres. Osmotic pressure is critical when dealing with blood cells. The amount of pressure required to stop the process of osmosis in your experimental set up.
The definition of osmotic pressure. Calculating osmotic pressure piimrt please urgently help. What is the osmotic pressure of a solution prepared from 137 g of the electrolyte hcl and enough water to make 0500l of solution at 18 degrees celsius.
 Osmotic Pressure This Example Problem Demonstrates How To
Osmotic Pressure This Example Problem Demonstrates How To
www.coursehero.com
 Docmd On Twitter 25 Osmotic Pressure Pressure Needed To
Docmd On Twitter 25 Osmotic Pressure Pressure Needed To
twitter.com
Https Abt Ucpress Edu Content Ucpabt 79 6 473 Full Text Pdf
 19 21 Previous 16 18 Next 22 23 Osmosis Low Osmotic
19 21 Previous 16 18 Next 22 23 Osmosis Low Osmotic
sites.google.com
 A Novel Ethanol Dehydration Process By Forward Osmosis Sciencedirect
A Novel Ethanol Dehydration Process By Forward Osmosis Sciencedirect
www.sciencedirect.com
 Based Upon The Technique Of Reverse Osmosis The Ap Toppr Com
Based Upon The Technique Of Reverse Osmosis The Ap Toppr Com
www.toppr.com
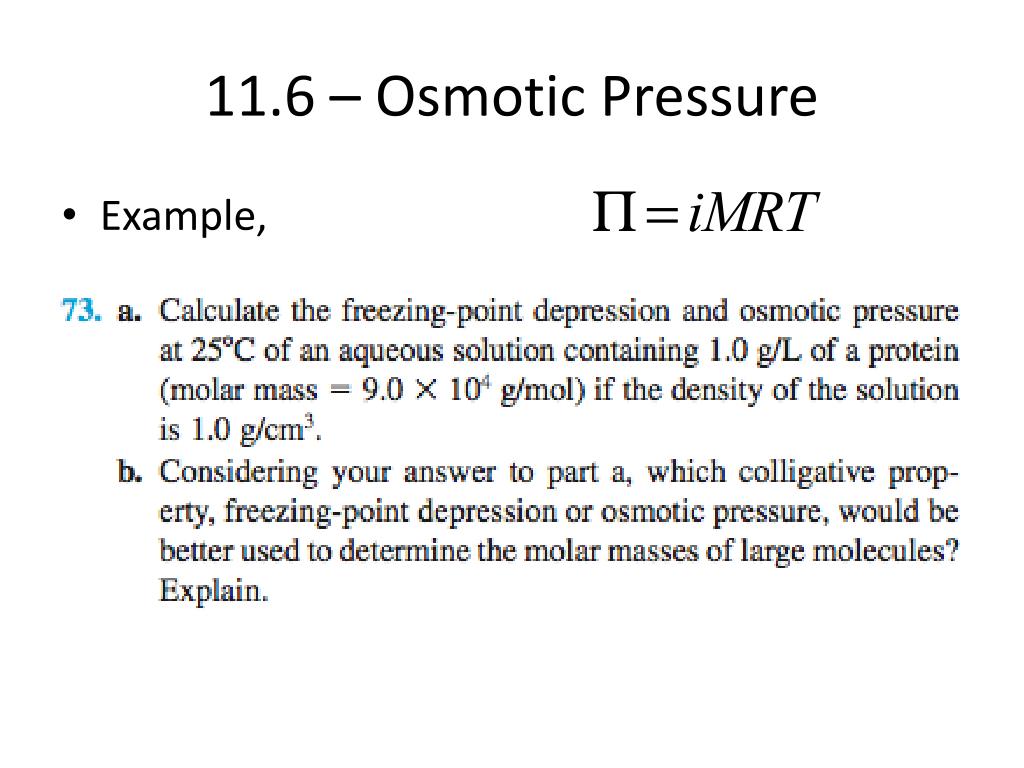 Ppt Chapter 11 Properties Of Solutions Powerpoint Presentation
Ppt Chapter 11 Properties Of Solutions Powerpoint Presentation
www.slideserve.com
 Osmotic Presure Help Mcat
Osmotic Presure Help Mcat
www.reddit.com
 The Osmotic Pressure Of Blood Is 7 7 Atm At 25 C What
The Osmotic Pressure Of Blood Is 7 7 Atm At 25 C What
socratic.org
This is such a great resource that you are providing and you give it away for free. I love seeing blog that understand the value of providing a quality resource for free. Bar to pounds per square inch
ReplyDelete