Alpha Beta And Gamma Radiation A Level
From wikibooks open books for an open world aqa a level physics. Alpha beta and gamma ionising radiation comes in three varieties.
Alpha Beta And Gamma Radiation Penetration Uses And Dangers
www.furryelephant.com
Alpha radiation consists of alpha particlesan alpha particle is identical to the nucleus of a helium atom which.
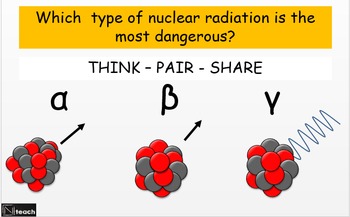
Alpha beta and gamma radiation a level. Gamma rays have no charge and so are unaffected by electric and magnetic fields. Therefore if the element is still in a higher energy state then the gamma particle emission occurs in order to obtain a lower energy level. All of these forms of radiation are energetic enough to pull electrons away from atoms.
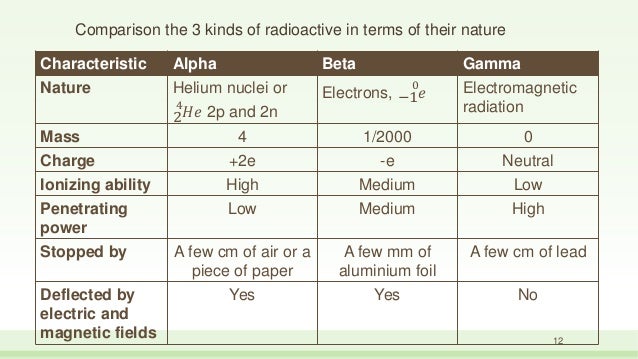
Alpha particles are made of four nucleons. Therefore they have a mass of approximately where so the mass of an alpha particle is 66410 27 kg 373 gevc 2. Since the gamma rays are in substance only a very high energy photons they are very penetrating matter and are thus biologically hazardous.
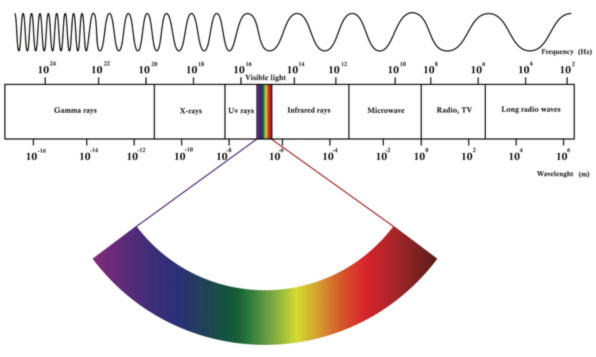
Gamma rays also known as gamma radiation refers to electromagnetic radiation no rest mass no charge of a very high energiesgamma rays are high energy photons with very short wavelengths and thus very high frequency. High speed electrons are able to knock off electrons from a material usually a metal. A alpha particles b beta particles g gamma rays.
Radioactivity and properties of alpha beta and gamma a level and ib physics. There is 1 pending change awaiting review. Aqa a level physicsalpha beta and gamma radiation.
Of radioactive atoms these are alpha beta and gamma radiation. Alpha beta and gamma radiation can be identified using the variation in their penetration. Just as gamma radiation is the excess energy of an excited nucleus after losing an alpha particle x ray radiation is the result of excited electrons.
Jump to navigation jump to search. The latest reviewed version was checked on 15 january 2018. The atoms that have had electrons removed in this way are now charged particles or ions and hence the name ionising radiation.
During radioactivity particles like alpha beta gamma rays are emitted by an atom due to unstable atom trying to gain stability. Difference between alpha beta and gamma particles definition. Covers the properties of alpha beta minus beta plus and gamma radiation including quark changes for beta.
X rays are identical to gamma rays although they originate from a different source. Hence the atoms eventually decay by emitting a particle that transforms when they are unstable and transforms the nucleus into a lower energy state. Alpha or beta decay may change the chemical element but cannot change the energy state of the element.
A photon of electromagnetic radiation. Identification using simple absorption experiments. Electrons and positrons which make up beta particles are antiparticles of each other.
An alpha particle is a. Mass of alpha beta and gamma radiation. The daughter nucleus can decay by emitting a gamma ray ie.
Properties Ionising Radiation Alpha Beta Gamma Ionizing
www.docbrown.info
 Ps1 8 Properties Of Alpha Beta And Gamma Radiation Uses Of
Ps1 8 Properties Of Alpha Beta And Gamma Radiation Uses Of
www.teacherspayteachers.com
 Physics Spm Chapter 5 Radioactivity
Physics Spm Chapter 5 Radioactivity
www.slideshare.net
 Marie Curie S Representation Of Alpha Beta And Gamma Rays Coming
Marie Curie S Representation Of Alpha Beta And Gamma Rays Coming
www.researchgate.net
Properties Ionising Radiation Alpha Beta Gamma Ionizing
www.docbrown.info
 How To Reduce Radiation Risk International Medcom Inc
How To Reduce Radiation Risk International Medcom Inc
medcom.com
What Is The Difference Between Alpha Beta And Gamma Radiation
www.quora.com
www.tes.com
0 Response to "Alpha Beta And Gamma Radiation A Level"
Post a Comment