Compare Alpha Beta And Gamma Radiation In Terms Of Properties
During radioactivity particles like alpha beta gamma rays are emitted by an atom due to unstable atom trying to gain stability. Given below is the table of characteristics of alpha beta and gamma radiations which also compares the charges and masses of the three rays and the above figure shows the penetration power of alpha beta and gamma rays.
Penetration Of Alpha Beta And Gamma Rays Pass My Exams Easy
www.passmyexams.co.uk
Hence the atoms eventually decay by emitting a particle that transforms when they are unstable and transforms the nucleus into a lower energy state.
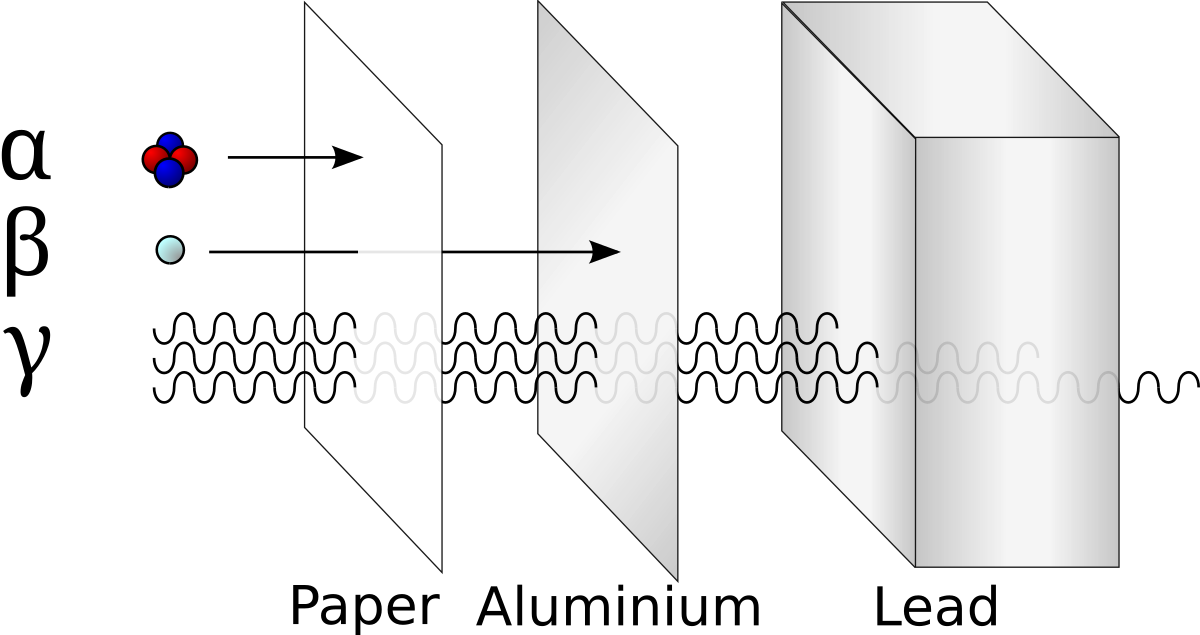
Compare alpha beta and gamma radiation in terms of properties. Main difference alpha vs beta vs gamma particles. Most of the properties of alpha beta and gamma particles have been already discussed. That is this type of radiation can cause an electrically neutral atom to lose.
A particle property of ionising radiation is its ability to ionise atoms. This decay occurs through emission of different particles. The emission of particles is also called the emission of radiationthe radiation is emitted from the nucleus of an atom converting protons or neutrons of the nucleus into different particles.
It naturally occurs when an unstable nucleus compare the difference between similar terms. Properties of alpha beta and gamma rays are alpha rays are positively charged particles with 2he4nucleus. When atoms decay they emit three types of radiation alpha beta and gamma.
These three different types of radiation have different properties. Properties of alpha beta and gamma radiation alpha particles beta particles and gamma rays all originate from the nucleus of a radioisotope. All three kinds of radiation are potentially hazardous to living tissue but some more than others as will be explained later on.
The alpha and beta radiation consist of actual matter that shoots off the atom while gamma rays are electromagnetic waves. Radioactivity is a process of decay of chemical elements with time. Alpha rays vs beta rays vs gamma rays compare alpha particles beta particles and gamma rays table an unstable atomic nuclei loss its energy by emitting radiations such as alpha rays beta rays and gamma rays by a process called radioactive decay.
Alpha beta vs gamma radiation a stream of energy quanta or particles with high energy is known as radiation. Beta rays are negatively charged particles also known as electrons. Alpha rays beta rays and gamma rays are the rays emitted during a substances radioactivity.
Their basic properties and differences were discussed in the article what are the three types of nuclear radiation. Of radioactive atoms these are alpha beta and gamma radiation. Alpha radiation consists of alpha particlesan alpha particle is identical to the nucleus of a helium atom which.
A substance with such an unstable nucleus is called the radioactive substance. Alpha beta and gamma radiation are three different types of nuclear radiation. Here we discuss the difference between alpha beta and gamma radiation.
 Worksheet Nuclear Decay Teacher Teacher Notes Name Key Class
Worksheet Nuclear Decay Teacher Teacher Notes Name Key Class
www.coursehero.com
 Radiation Penetrating Power Of Radiation Students Britannica
Radiation Penetrating Power Of Radiation Students Britannica
kids.britannica.com
 Modes Of Radioactive Decay Introduction To Chemistry
Modes Of Radioactive Decay Introduction To Chemistry
courses.lumenlearning.com
 Radiation Wikipedia
Radiation Wikipedia
en.wikipedia.org
Difference Between Alpha Beta And Gamma Particles Definition
pediaa.com
 Cosmic Ray Wikipedia
Cosmic Ray Wikipedia
en.wikipedia.org
What Are Alpha Beta And Gamma Rays Quora
www.quora.com
 How To Reduce Radiation Risk International Medcom Inc
How To Reduce Radiation Risk International Medcom Inc
medcom.com
 Chapter 2 Properties Of Radiations And Radioisotopes
Chapter 2 Properties Of Radiations And Radioisotopes
www.slideshare.net
0 Response to "Compare Alpha Beta And Gamma Radiation In Terms Of Properties"
Post a Comment