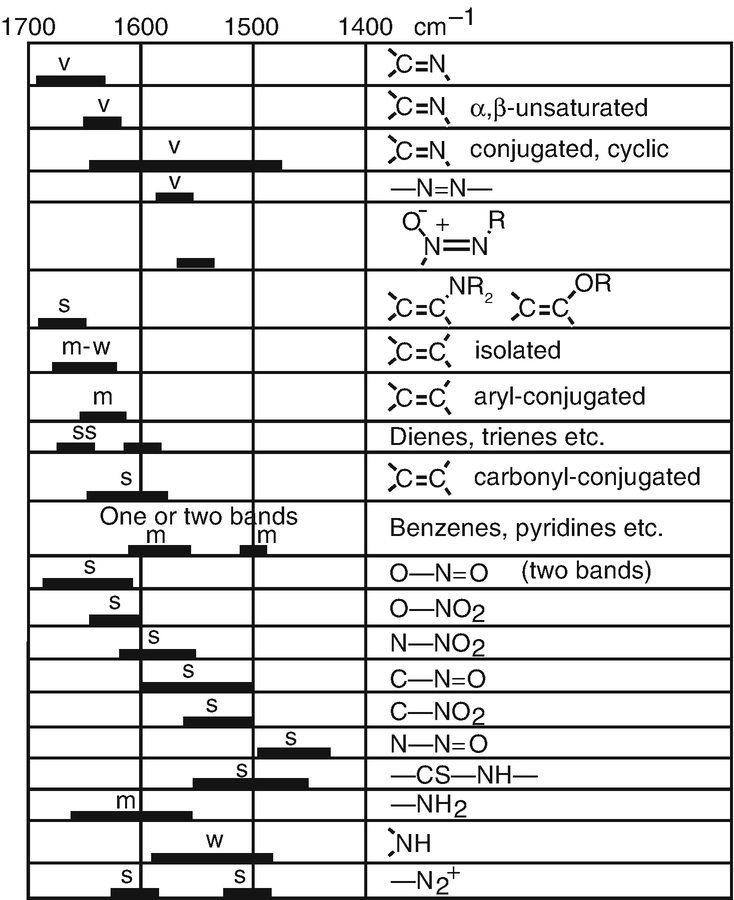
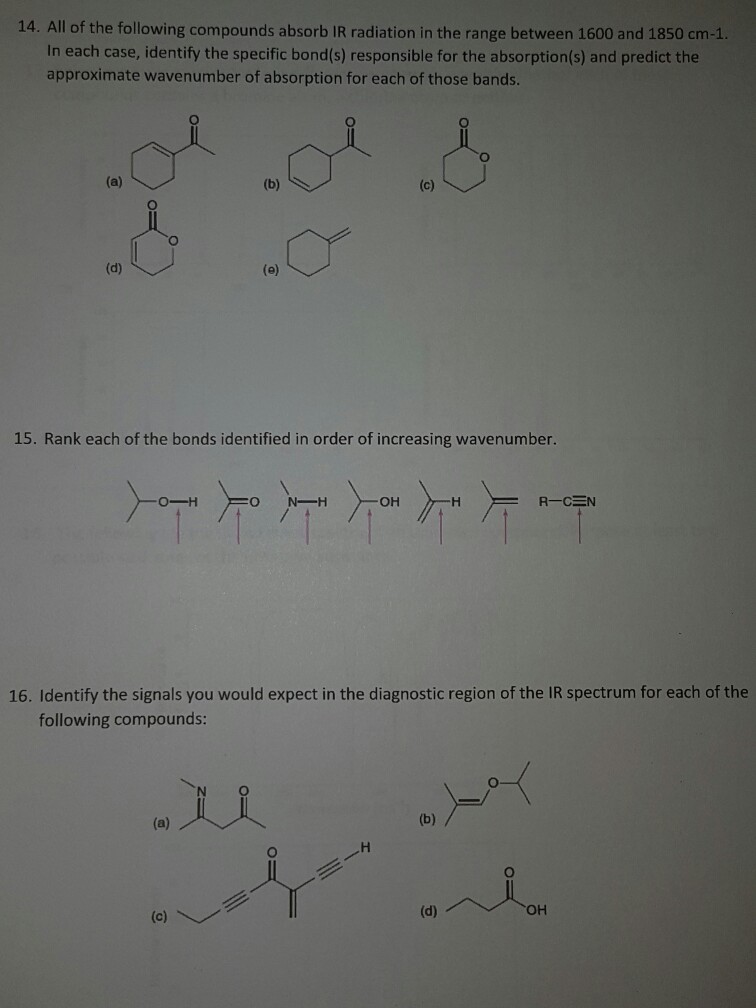
All Of The Following Compounds Absorb Infrared Radiation Between 1600 And 1800 Cm1
The portion of the infrared region most useful for analyses of organic compounds is 4000 666 cm 1 photon energies associated with this part of the infrared are not large enough to excite electrons the covalent bonds in molecules a b are not rigid sticks or rods but are more like stiff springs that can be stretched and bent. All of the following compounds absorb infrared radiation between 1600 and 1800 cm 1 in each case 1.
 Infrared And Raman Spectra Springerlink
Infrared And Raman Spectra Springerlink
link.springer.com
The ir spectrum of a sample is a graph of how strongly a compound absorbs ir radiation of different wavenumbers or frequencies.

All of the following compounds absorb infrared radiation between 1600 and 1800 cm1. An important observation made by early researchers is that many functional group absorb infrared radiation at about the same wavenumber regardless of the structure of the rest of the molecule. A 1 in the above compound. The ir absorptions of the following compounds can be determined as.
In each case identify the specific bonds responsible for the absorptions and predict the approximate wavenumber of absorption for each of those bonds. All of the following compounds absorb ir radiation in the range between 1600 and 1850 cm 1. 3 the functional group of the compound which has the greater value of the ir absorption due to greater dipole moment has the stronger absorption of ir radiation.
Consequently virtually all organic compounds will absorb infrared radiation that corresponds in energy to these vibrations. The following compound absorbs ir radiation in the range between 1600 and 1850 cm 1. Virtually all organic compounds absorb ir radiation.
The frequency absorbed varies with the functional groups present eg oh nh co cc etc. Many molecules absorb infrared radiation causing the bonds in molecules to bend and stretch. Predict which compound of each pair absorbs more strongly in this region.
2 the ir absorptions of the above compounds are. All of the following compounds absorb infrared radiation between 1600 and 1800 cm 1. Predict which compound of each pair absorbs more strongly in this region.
Predict the approximate absorption frequencies. Between 1400 and 1050 cm 1 all amides show. Select the specific bonds responsible for the absorption sand the approximate wave number of absorption for each of those bonds.
Infrared spectrometers similar in principle to the uv visible spectrometer described elsewhere permit chemists to obtain absorption spectra of compounds that are a unique reflection of their molecular structure. In each case 10 points show which bonds absorb in this region predict the approximate absorption frequencies. Show which bonds absorb in this region.
For example c h stretching vibrations usually appear between 3200 and 2800cm 1 and carbonylco stretching vibrations usually appear between 1800 and.
 Organic Chemistry I Ii Reading Assignment Infra Red
Organic Chemistry I Ii Reading Assignment Infra Red
tophat.com
 Infrared Spectroscopy In Analysis Of Plastics Recycling
Infrared Spectroscopy In Analysis Of Plastics Recycling
onlinelibrary.wiley.com
 Mid Infrared Spectroscopy Anomalies Artifacts And Common Errors
Mid Infrared Spectroscopy Anomalies Artifacts And Common Errors
onlinelibrary.wiley.com
Http Www Ifsc Usp Br Lavfis2 Bancoapostilasimagens Apluminescencia Infrared 20spectroscop1 Pdf
 Library Based Identification And Characterisation Of Polymers With
Library Based Identification And Characterisation Of Polymers With
pubs.rsc.org
www.chegg.com
www.chegg.com
 Infrared Spectroscopy Fundamentals And Applications By Barbara Stuart
Infrared Spectroscopy Fundamentals And Applications By Barbara Stuart
www.slideshare.net
0 Response to "All Of The Following Compounds Absorb Infrared Radiation Between 1600 And 1800 Cm1"
Post a Comment