Electromagnetic Radiation Photons Energy
It always moves at the speed of light in a vacuum. Equivalently the longer the photons wavelength the lower its energy.
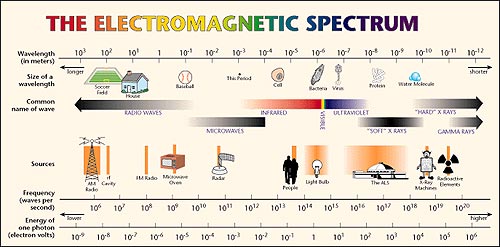
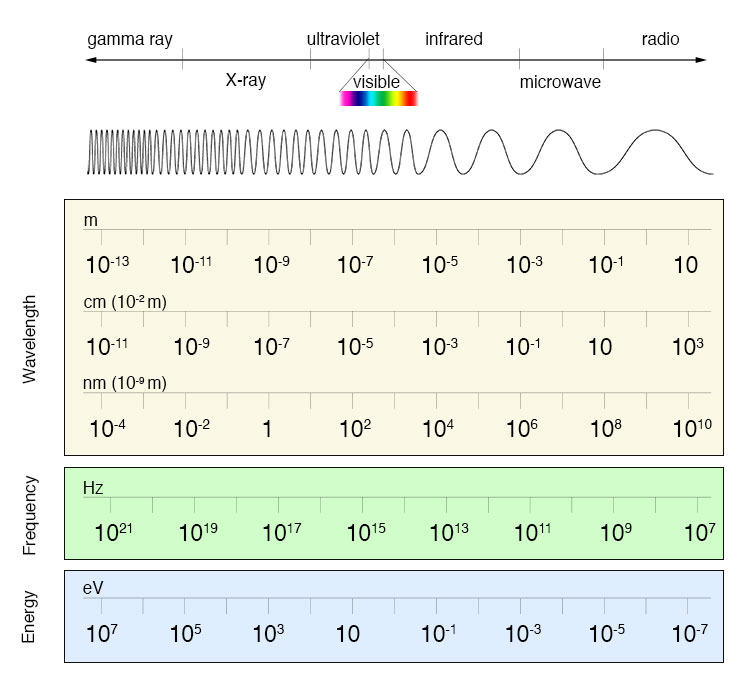 Electromagnetic Spectrum
Electromagnetic Spectrum
imagine.gsfc.nasa.gov
Electromagnetic radiation is composed of electromagnetic waves with synchronized oscillations of electric and magne.
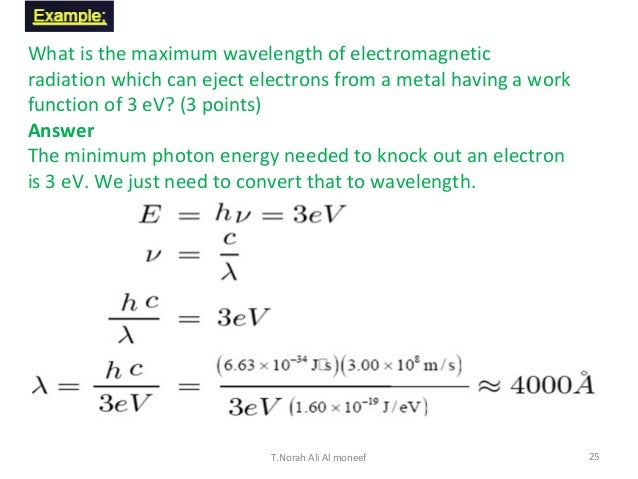
Electromagnetic radiation photons energy. Radio waves gamma rays visible light and all the other parts of the electromagnetic spectrum are electromagnetic radiation. Example 1 calculate the energy of a photon of radiation whose frequency is 500 1014 hz. You use either the formula e hf or e hcl.
Many other kinds of ionizing radiation are made of non em particles. Gamma rays a form of nuclear and cosmic em radiation can have the highest frequencies and hence the highest photon energies in the em spectrumfor example a g ray photon with f 10 21 hz has an energy e hf 663 10 13 j 414 mev. This is sufficient energy to ionize thousands of atoms and molecules since only 10 to 1000 ev are needed per ionization.
Electromagnetic radiation can be described in terms of a stream of mass less particles called photons each traveling in a wave like pattern at the speed of light. Like all elementary particles photons are. A notable effect attenuation is to gradually reduce the intensity of light waves as they propagate through a medium.
The photon is a type of elementary particleit is the quantum of the electromagnetic field including electromagnetic radiation such as light and radio waves and the force carrier for the electromagnetic force even when static via virtual particlesthe invariant mass of the photon is zero. Each photon contains a certain amount of energy. H is plancks constant f is the frequency c is the speed of light and l is the wavelength of the radiation.
Electromagnetic radiation composed of photons that carry minimum ionization energy or more which includes the entire spectrum with shorter wavelengths is therefore termed ionizing radiation. Photon energy is the energy carried by a single photonthe amount of energy is directly proportional to the photons electromagnetic frequency and thus equivalently is inversely proportional to the wavelengththe higher the photons frequency the higher its energy. Electromagnetic radiation is a form of energy that includes radio waves microwaves x rays and gamma rays as well as visible light.
In physics absorption of electromagnetic radiation is how matter typically electrons bound in atoms takes up a photons energy and so transforms electromagnetic energy into internal energy of the absorber for example thermal energy. Electromagnetic radiation is a form of energy that is in the form of light and travels at the speed of light3108 ms in vacuum.
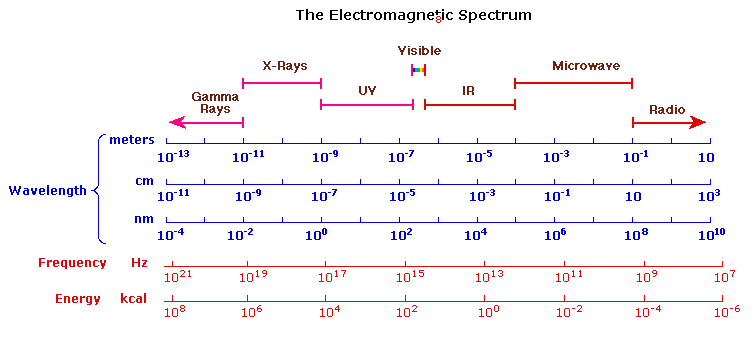 Uv Visible Spectroscopy
Uv Visible Spectroscopy
www2.chemistry.msu.edu
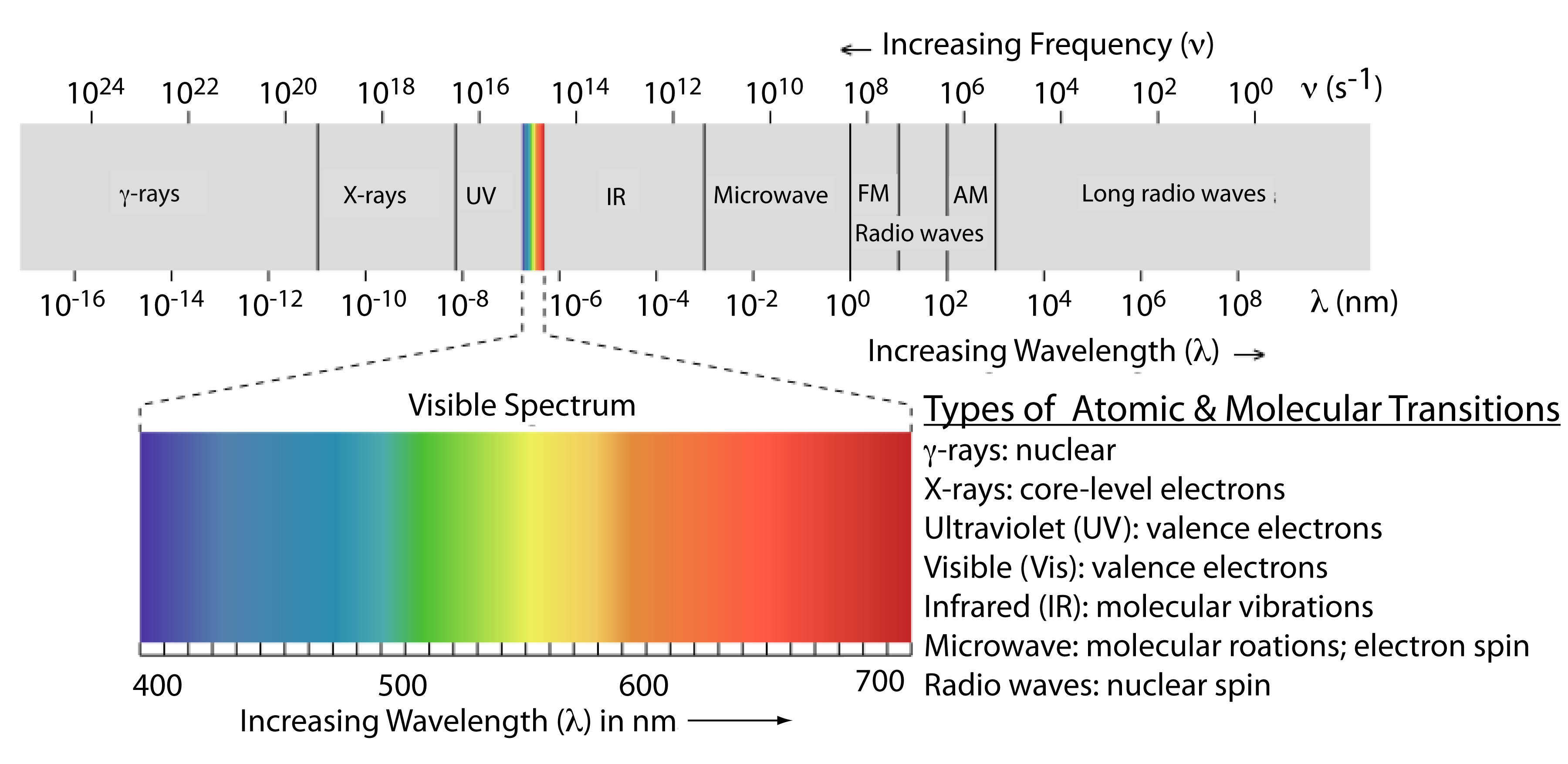 13 1 The Electromagnetic Spectrum Chemistry Libretexts
13 1 The Electromagnetic Spectrum Chemistry Libretexts
chem.libretexts.org
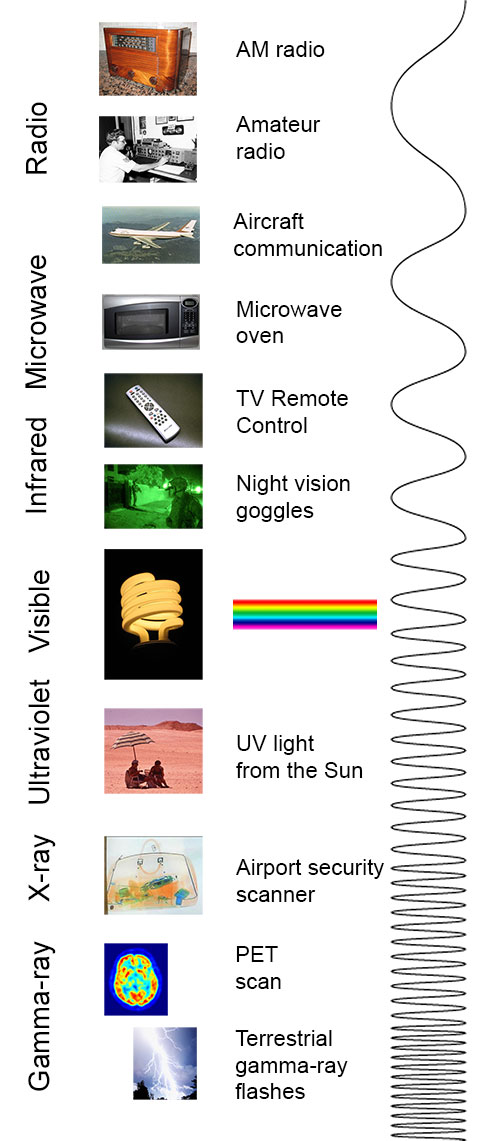 Electromagnetic Spectrum Introduction
Electromagnetic Spectrum Introduction
imagine.gsfc.nasa.gov
 Nature Of Radiation
Nature Of Radiation
www.nde-ed.org
 Electromagnetic Radiation An Overview Sciencedirect Topics
Electromagnetic Radiation An Overview Sciencedirect Topics
www.sciencedirect.com
 Light Hydrogen Energy Levels Naap
Light Hydrogen Energy Levels Naap
astro.unl.edu
Electromagnetic Radiation
www.astronomynotes.com
 Synchrotron Radiation Wikipedia
Synchrotron Radiation Wikipedia
en.wikipedia.org
0 Response to "Electromagnetic Radiation Photons Energy"
Post a Comment