Electromagnetic Radiation Photon Frequency
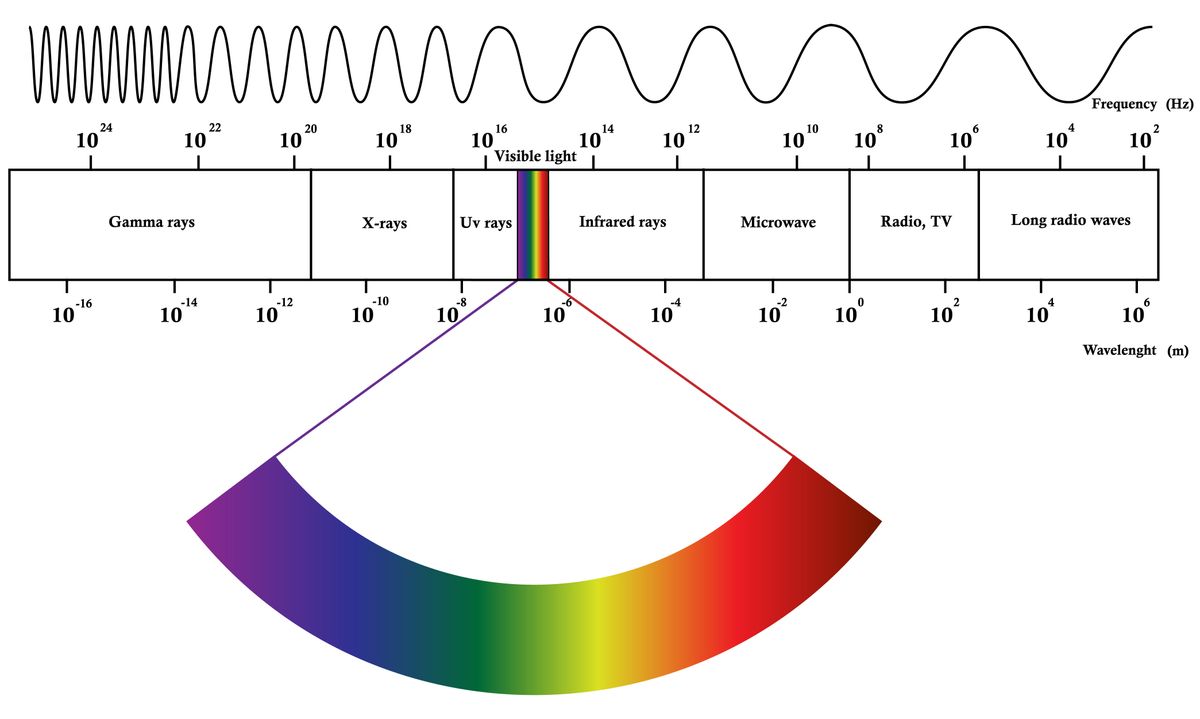
The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from below one hertz to above 10 25 hertz corresponding to wavelengths from thousands of kilometers down to a fraction of the size of an atomic nucleus. X ray photons with a wavelength of 0.
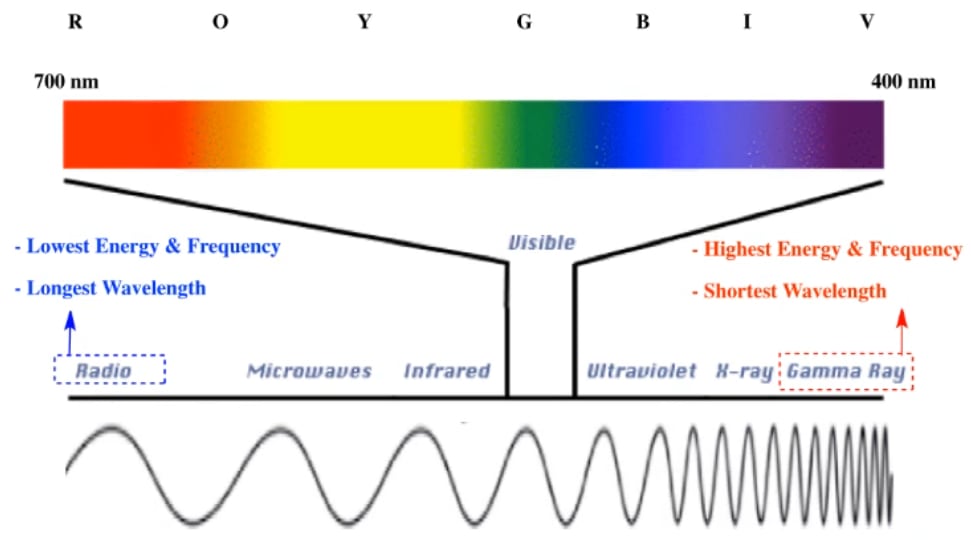 Electromagnetic Spectrum Chemistry Video Clutch Prep
Electromagnetic Spectrum Chemistry Video Clutch Prep
www.clutchprep.com
It always moves at the speed of light in a vacuum.

Electromagnetic radiation photon frequency. Like all elementary particles photons are. Calculate the energy of a photon of electromagnetic radiation whose frequency is 2901014 s1 calculate the energy of a photon of radiation whose wavelength is 413 nm what wavelength of radiation has photons of energy 6031019 j. As a wave it is represented by velocity wavelength and frequency.
The types of interaction can range from electronic excitation to molecular vibration. The electromagnetic spectrum includes light with a range of frequencies wavelengths and energies. Equivalently the longer the photons wavelength the lower its energy.
Electromagnetic radiation is a form of energy that includes radio waves microwaves x rays and gamma rays as well as visible light. 1023mhz typical frequency for fm radio broadcasting 1035khz typical frequency for am radio broadcasting assume four significant figures 8356 mhz common frequency used for cell phone how much energy is contained in 1 mol of each of the following. The effects of electromagnetic radiation upon living cells including those in humans depends upon the radiations power and frequency.
Light quanta are typically described by frequency f wavelength l or photon energy e. The spectrum can be ordered according to frequency or wavelength. Electromagnetic radiation interacts with matter in different ways in different parts of the spectrum.
Calculate the energy of a photon of electromagnetic radiation at each of the following frequencies. Light is an em wave since the speed of em waves is the same as the speed of light. Photon energy is the energy carried by a single photonthe amount of energy is directly proportional to the photons electromagnetic frequency and thus equivalently is inversely proportional to the wavelengththe higher the photons frequency the higher its energy.
The photon is a type of elementary particleit is the quantum of the electromagnetic field including electromagnetic radiation such as light and radio waves and the force carrier for the electromagnetic force even when static via virtual particlesthe invariant mass of the photon is zero. Electromagnetic radiation can either acts as a wave or a particle a photon. The electromagnetic spectrum is the range of frequencies the spectrum of electromagnetic radiation and their respective wavelengths and photon energies.
As the wavelength of electromagnetic radiation the frequency. For low frequency radiation radio waves to visible light the best understood effects are those due to radiation power alone acting through heating when radiation is absorbed. Light is electromagnetic radiation composed of electric and magnetic field components.
Climate Science Investigations South Florida Energy The Driver
www.ces.fau.edu
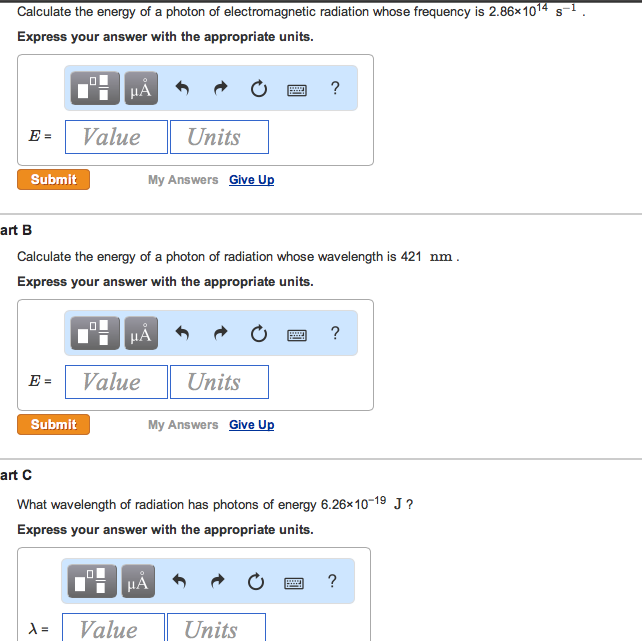 Solved Calculate The Energy Of A Photon Of Electromagneti
Solved Calculate The Energy Of A Photon Of Electromagneti
www.chegg.com
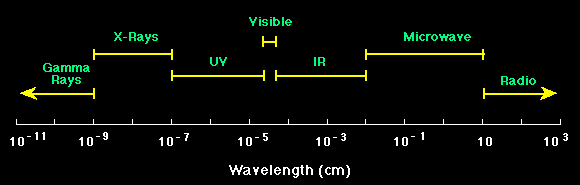 Electromagnetic Spectrum
Electromagnetic Spectrum
web.pa.msu.edu
 Physics Class 12 Ncert Solutions Chapter 8 Electromagnetic Waves
Physics Class 12 Ncert Solutions Chapter 8 Electromagnetic Waves
www.flexiprep.com
 Lesson 6 Electromagnetic Waves Lesson 1
Lesson 6 Electromagnetic Waves Lesson 1
www.yumpu.com
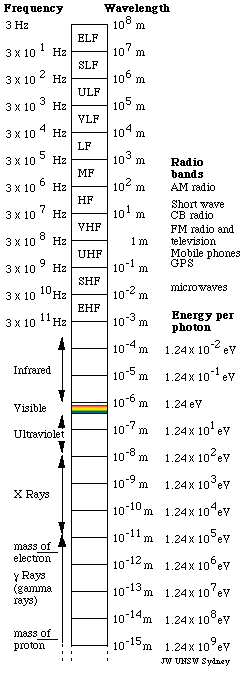 The Electromagnetic Spectrum
The Electromagnetic Spectrum
www.animations.physics.unsw.edu.au
Https Wwphs Sharpschool Com Common Pages Displayfile Aspx Itemid 14794206
 What Is Electromagnetic Radiation Live Science
What Is Electromagnetic Radiation Live Science
www.livescience.com
0 Response to "Electromagnetic Radiation Photon Frequency"
Post a Comment