All About Characteristic Radiation
X ray radiation is uncharged neutral particle an electron with kinetic energy eo may interact with the atoms of the target by ejecting an orbital electron such as a k l or m electron. Explain what the electromagnetic spectrum is and how scientists use it.
 Physical Principles Of Ionizing Radiations
Physical Principles Of Ionizing Radiations
www.ceessentials.net
Looking for characteristic radiation.

All about characteristic radiation. Characteristic x rays are emitted from heavy elements when their electrons make transitions between the lower atomic energy levels. After reading this section you will be able to do the following. The intensity of a characteristic x ray spectrum both primary and fluorescent depends on the probability p r of a radiation transition in the atom having the vacancy in the ith level.
So far we have learned about the atom the phenomenon of radioactivity and we have looked at both nuclear reactions and x ray generation. The energies of characteristic photons are a function of the energy levels of various electron orbital levels and hence are characteristic of the target atoms. Characteristic x rays are emitted when outer shell electrons fill a vacancy in the inner shell of an atom releasing x rays in a pattern that is characteristic to each element.
The characteristic x ray emission which is shown as two sharp peaks in the illustration at left occur when vacancies are produced in the n1 or k shell of the atom and electrons drop down from above to fill the gap. Monochromatic radiation that is produced when an electron is ejected from an atom and another takes its place by jumping from another shell. Acute lymphoblastic leukemia all is a cancer of the lymphoid line of blood cells characterized by the development of large numbers of immature lymphocytes.
Symptoms may include feeling tired pale skin color fever easy bleeding or bruising enlarged lymph nodes or bone pain. In this channel of youtube are edited videos for high school students as well as for students of physics chemistry biology medicine pharmacy agriculture and all branches studying science of. Characteristic radiation from the k shell occurs only above 70 kvp with a tungsten target and occurs as discrete increments compared with bremsstrahlung radiation.
Find out information about characteristic radiation. The energy of the emitted photon is the difference between that of the two shell positions. Characteristic x rays were discovered by charles glover barkla in 1909 who later won the nobel prize in physics for his discovery in 1917.
Radiation originating in an atom following removal of an electron whose wavelength depends only on the element concerned and the energy levels involved explanation of characteristic radiation. Using the continous radiation of an x ray tube with a target consisting mostly of heavy elements it is possible to excite x ray fluorescence.
Bremsstrahlung And Characteristic Interactions
 Characteristic Radiation In Tungsten Targets Shel L Of Electrons
Characteristic Radiation In Tungsten Targets Shel L Of Electrons
slideplayer.com
 X Ray Spectra Continuous And Characteristic X Ray Spectra
X Ray Spectra Continuous And Characteristic X Ray Spectra
www.brainkart.com
 Electron Microscopy Proceedings Of The Stockholm Conference
Electron Microscopy Proceedings Of The Stockholm Conference
www.alamy.com
X Rays
hyperphysics.phy-astr.gsu.edu
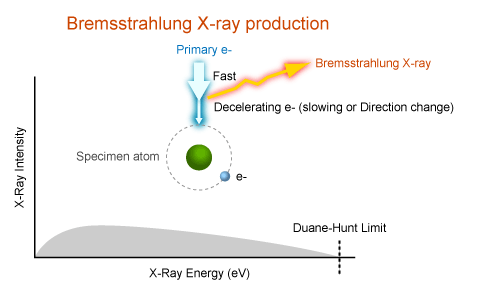 Bremsstrahlung X Ray Generation Myscope
Bremsstrahlung X Ray Generation Myscope
myscope.training
 Production Of X Rays
Production Of X Rays
www.radiologycafe.com
X Ray Image Formation And Contrast
www.sprawls.org
Chubu Electric Power Co Inc Characteristics Of Radiation And
hamaoka.chuden.jp
0 Response to "All About Characteristic Radiation"
Post a Comment