All About Radioactivity In Chemistry
It is in essence an attribute of individual atomic nuclei. Some radioactive isotopes also occur in elements with lower atomic numbers.
 Phenomenon Of Radioactivity In Hindi Hindi Complete Course
Phenomenon Of Radioactivity In Hindi Hindi Complete Course
unacademy.com
The si unit of radioactivity is the becquerel bq.

All about radioactivity in chemistry. Radioactivity is the process by which an unstable atomic nucleus loses energy by emitting radiation. In 1896 henri becquerel first observed the phenomena of radioactivity but the term radioactivity was coined by marie curie. Marie curie discovered the radioactive elements namely polonium and radium in 1898.
These short video and text based lessons outline the properties. Nuclear chemistry is the sub field of chemistry dealing with radioactivity nuclear processes and transformations in the nuclei of atoms such as nuclear transmutation and nuclear properties. Bismuth is the heaviest element with at least one stable isotope.
All elements heavier than bismuth are radioactive. Activities take a ten question quiz about this page. Radioactivity was discovered by the scientist a.
While radioactivity results in the release of radiation not all radiation is produced by radioactive material. This video is all about radioactivity if any body find any thing wrong please comment so we can improve in our next video telegram link for our channel is given below rrb je 2019 complete study. Radioactivity in chemistry chapter summary.
Radioactive decay is a property of several naturally occurring elements as well as of artificially produced isotopes of the elements. Positron a particle with the same mass as an electron but a charge of 1 instead of 1 emission isnt observed in natural radioactivity but it is a common mode of decay in induced radioactivity. It is the chemistry of radioactive elements such as the actinides radium and radon together with the chemistry associated with equipment such as nuclear reactors which are designed to perform nuclear.
Likewise radioactivity is a nuclear phenomenon that happens naturally because of the nuclear instability of atoms. Radioactive isotopes are prepared in the lab using bombardment reactions to convert a stable nucleus into one which is radioactive. All elements with more than 83 protons ie an atomic number 1 greater than 83 are radioactive.
Radioactivity radioactivity originates from extraterrestrial sources and terrestrial geologic sources. Work through this chapters engaging lessons to review radioactivity in chemistry. Radioactivity definition the phenomenon exhibited by and being a property of certain elements of spontaneously emitting radiation resulting from changes in the nuclei of atoms of the element.
Other units include the curie gray and sievert. Radioactivity property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously.
 Nuclear Chemistry Radioactivity Cheat Sheet Online Chemistry
Nuclear Chemistry Radioactivity Cheat Sheet Online Chemistry
www.chemistrytutorials.org
 Radioactivity Definition Types Uses Video Lesson
Radioactivity Definition Types Uses Video Lesson
study.com
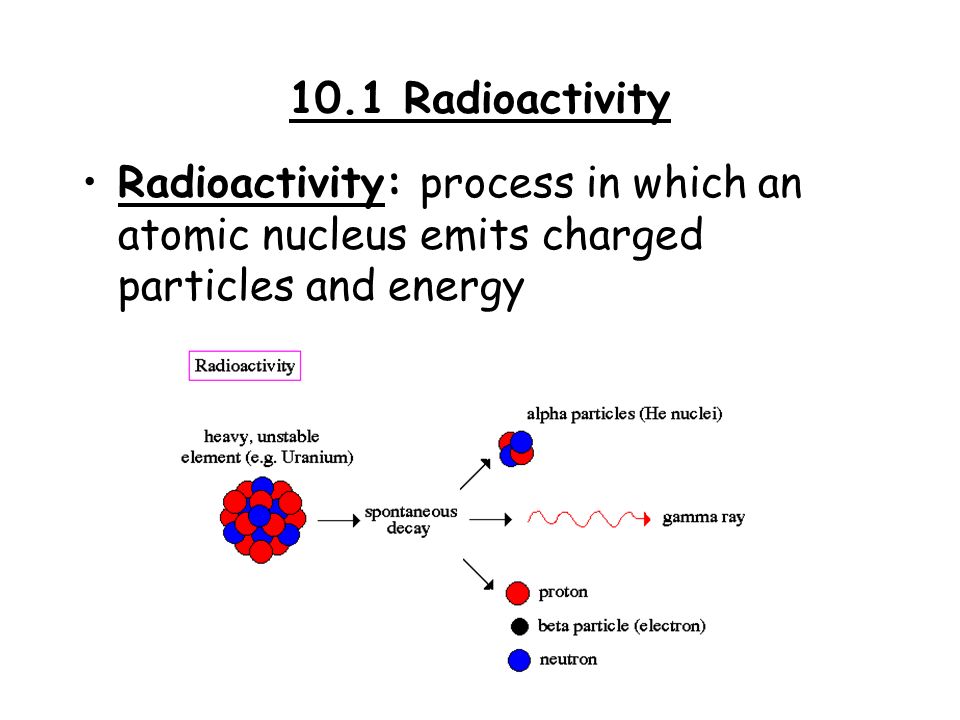 Nuclear Chemistry Ppt Video Online Download
Nuclear Chemistry Ppt Video Online Download
slideplayer.com
 Nuclear Chemistry Radioactivity Radiation Alpha Beta Gamma
Nuclear Chemistry Radioactivity Radiation Alpha Beta Gamma
www.youtube.com
 Copyright 2011 Pearson Education Inc Chapter 19 Radioactivity
Copyright 2011 Pearson Education Inc Chapter 19 Radioactivity
slideplayer.com
 Ppt Nuclear Chemistry Powerpoint Presentation Free Download
Ppt Nuclear Chemistry Powerpoint Presentation Free Download
www.slideserve.com
 Radioactive Decay Definition Formula Types Video Lesson
Radioactive Decay Definition Formula Types Video Lesson
study.com
 Chemical And Physical Properties Radioactivity Radioisotopes
Chemical And Physical Properties Radioactivity Radioisotopes
www.slideshare.net
0 Response to "All About Radioactivity In Chemistry"
Post a Comment