Alpha Beta And Gamma Rays Difference
Here we discuss the difference between alpha beta and gamma radiation. Beta ray also referred to as beta radiation can be described as a high energy high speed electron positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay.
Difference Between Alpha Beta And Gamma Radiation
pediaa.com
These three different types of radiation have different properties.
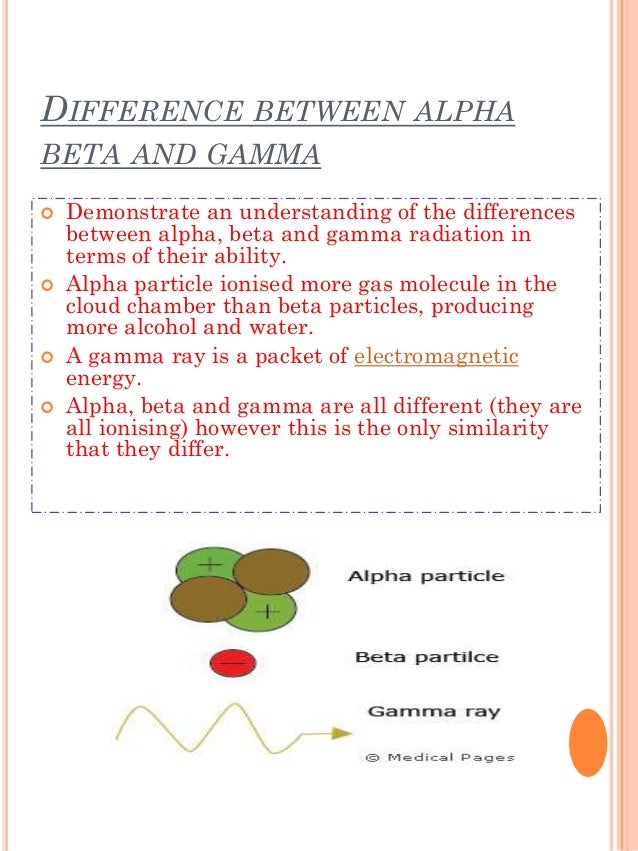
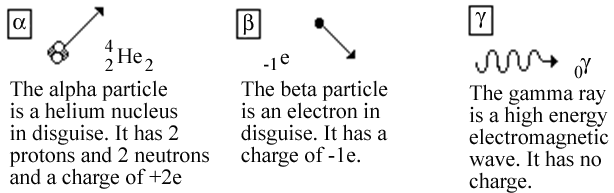
Alpha beta and gamma rays difference. It is positively charged rays. Gamma radiations are high energy radiations that are in the form of electromagnetic waves and these radiations do not give off any particle like alpha and gamma. Alpha particles are he 4 nuclei and beta is either electrons or positrons.
This decay occurs through emission of different particles. Alpha and beta radiation are stream of particles consisting mass. These are known as alpha beta and gamma rays.
Gamma radiation is an electromagnetic radiation and consists of high energy quanta. It has much ionising power than beta rays. During radioactivity particles like alpha beta gamma rays are emitted by an atom due to unstable atom trying to gain stability.
Alpha beta and gamma radiation are three different types of nuclear radiation. The mass of alpha paritcle is much greater than beta particles. Alpha rays vs beta rays vs gamma rays compare alpha particles beta particles and gamma rays table an unstable atomic nuclei loss its energy by emitting radiations such as alpha rays beta rays and gamma rays by a process called radioactive decay.
Alpha ray is particle of helium nuclei. Beta rays have a mass which is half of one thousand of the mass of a proton and carry either a single negative electron or positive positron charge. It has velocity 110th to 1100th the velocity of ligth.
Radioactivity is a process of decay of chemical elements with time. It has less penetrating power than beta a nd gamma. The emission of particles is also called the emission of radiationthe radiation is emitted from the nucleus of an atom converting protons or neutrons of the nucleus into different particles.
Hence the atoms eventually decay by emitting a particle that transforms when they are unstable and transforms the nucleus into a lower energy state. Main difference alpha vs beta vs gamma particles. Beta radiation is the producer of fast moving electrons and can penetrate further in comparison to the alpha particles.
It is having charge 2e. What is the difference between alpha beta and gamma radiation. Alpha radiation can be described as the producer of high energy and fast moving helium particles.
Alpha rays are helium 4 nuclei beta rays can be electrons or positrons gamma rays are high energy electromagnetic waves. A substance with such an unstable nucleus is called the radioactive substance. Their basic properties and differences were discussed in the article what are the three types of nuclear radiation.
 Alpha Beta Gamma Radiation Chart Sarta Innovations2019 Org
Alpha Beta Gamma Radiation Chart Sarta Innovations2019 Org
sarta.innovations2019.org
Gcse Nuclear Radiation Types Of Radioactivity
www.darvill.clara.net
 Properties Of Alpha Beta And Gamma Rays And Differences
Properties Of Alpha Beta And Gamma Rays And Differences
byjus.com
Nuclear Reactions
perso.numericable.fr
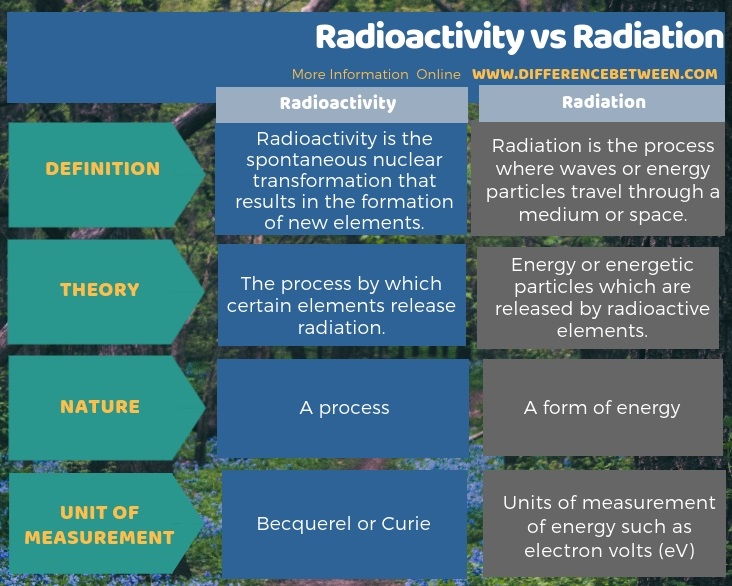 Difference Between Radioactivity And Radiation Compare The
Difference Between Radioactivity And Radiation Compare The
www.differencebetween.com
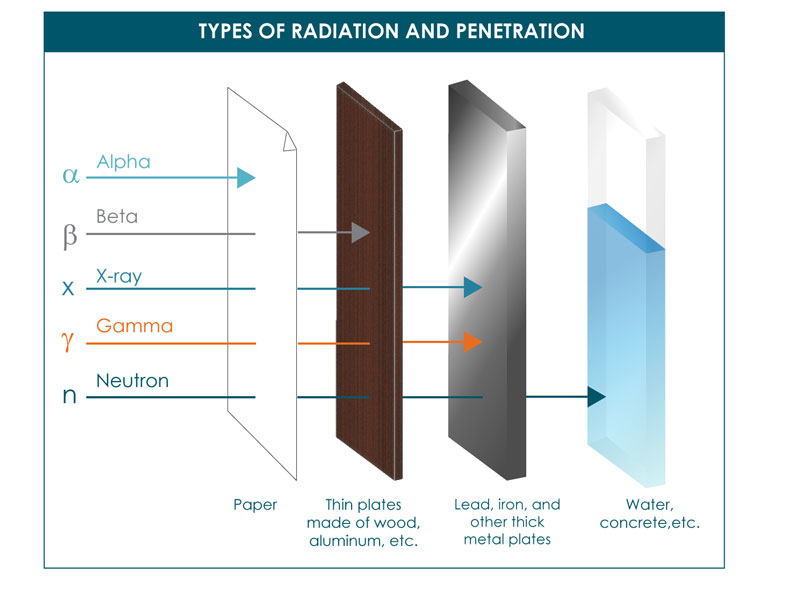 Alpha Beta Gamma Torun Rsd7 Org
Alpha Beta Gamma Torun Rsd7 Org
torun.rsd7.org
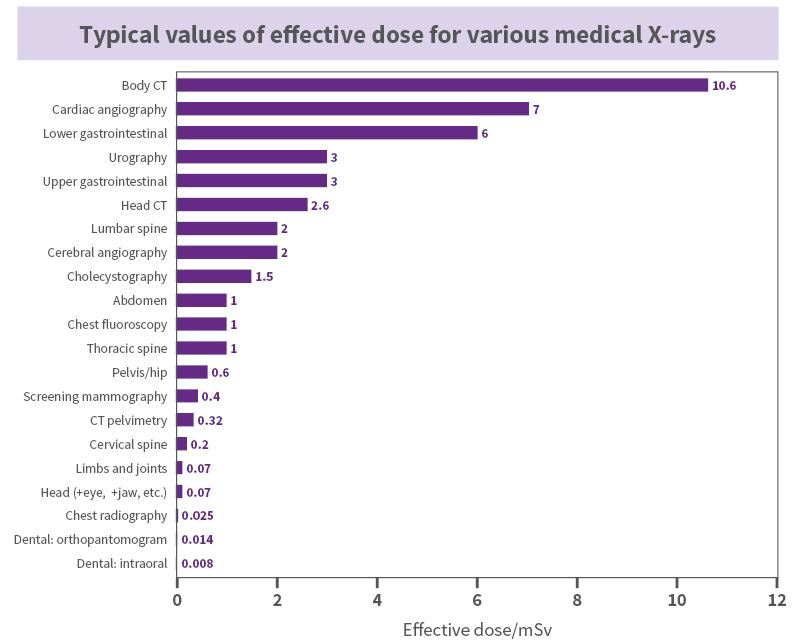 X Rays Arpansa
X Rays Arpansa
www.arpansa.gov.au
 Science Alpha Beta Gamma
Science Alpha Beta Gamma
www.slideshare.net
0 Response to "Alpha Beta And Gamma Rays Difference"
Post a Comment