Electromagnetic Radiation Photon Wavelength
Light is an em wave since the speed of em waves is the same as the speed of light. Each photon contains a certain amount of energy.

www.chegg.com
Radio waves gamma rays visible light and all the other parts of the electromagnetic spectrum are electromagnetic radiation.
Electromagnetic radiation photon wavelength. When electromagnetic radiation interacts with single atoms and molecules its behavior also depends on the amount of energy per quantum photon it carries. Wave number 1. Electromagnetic radiation is a form of energy that propagates as both electrical and magnetic waves traveling in packets of energy called photons.
The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from below one hertz to above 10 25 hertz corresponding to wavelengths from thousands of kilometers down to a fraction of the size of an atomic nucleus. The behavior of electromagnetic radiation depends on its wavelength. These will make many calculations a little easier.
Electromagnetic radiation can either acts as a wave or a particle a photon. Since many wavelengths are stated in nanometers nm it is also useful to know that hc 1240 ev nm. All em radiation is composed of photons.
A21 describe the electromagnetic spectrum ib chemistry sl youtube this time with equations. The discovery of infrared radiation is ascribed to astronomer william herschel who published his results in 1800 before the royal society of london. As a wave it is represented by velocity wavelength and frequency.
There is a spectrum of electromagnetic radiation with variable wavelengths and frequency which in turn imparts different characteristics. Electromagnetic radiation of wavelengths other than those of visible light were discovered in the early 19th century. The electromagnetic spectrum is the range of frequencies the spectrum of electromagnetic radiation and their respective wavelengths and photon energies.
Electromagnetic radiation can be described in terms of a stream of mass less particles called photons each traveling in a wave like pattern at the speed of light. Figure 1 shows various divisions of the em spectrum plotted against wavelength frequency and photon energy.
 Interconverting Wavelength Frequency And Photon Energy Youtube
Interconverting Wavelength Frequency And Photon Energy Youtube
www.youtube.com
 Electromagnetic Radiation An Overview Sciencedirect Topics
Electromagnetic Radiation An Overview Sciencedirect Topics
www.sciencedirect.com
Electromagnetic Force Multiwavelength Astronomy
ecuip.lib.uchicago.edu
:max_bytes(150000):strip_icc()/what-is-the-rydberg-formula-604285_final-251d1441e24e44c88aab687409554ed4.png) What Is The Rydberg Formula And How Does It Work
What Is The Rydberg Formula And How Does It Work
www.thoughtco.com
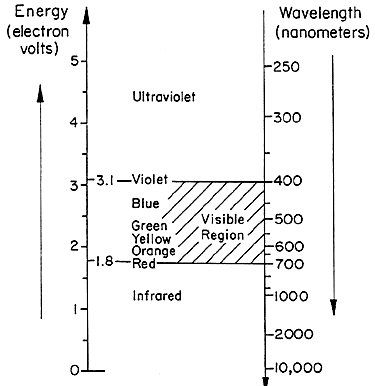 Reading On Color Light Part I
Reading On Color Light Part I
www.asu.edu
 My First Pages 1 2 Text Version Fliphtml5
My First Pages 1 2 Text Version Fliphtml5
fliphtml5.com
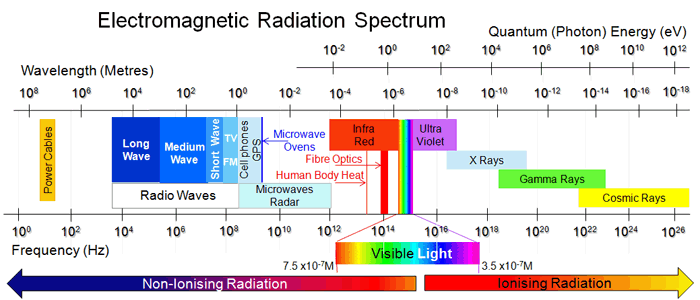 Electromagnetic Radiation And Radio Waves
Electromagnetic Radiation And Radio Waves
www.mpoweruk.com
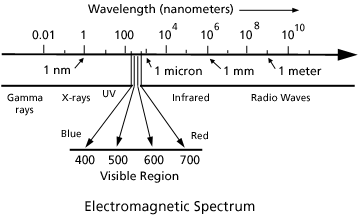 Reading On Color Light Part I
Reading On Color Light Part I
www.asu.edu
0 Response to "Electromagnetic Radiation Photon Wavelength"
Post a Comment