Alpha Beta And Gamma Radiation Are Distinguished By Their Charge
Describes of the properties of alpha particle radiation beta particle radiation and gamma radiation in terms of their change mass penetration of materials behaviour in an electric field the relative ionising capacity and the dangers of ionising radiation from both external radioactive sources radioisotopes and internally ingested radionuclide. A nucleus emit these different particles in order to become stable.
 Gamma Radiation An Overview Sciencedirect Topics
Gamma Radiation An Overview Sciencedirect Topics
www.sciencedirect.com
Electrons and positrons which make up beta particles are antiparticles of each other.

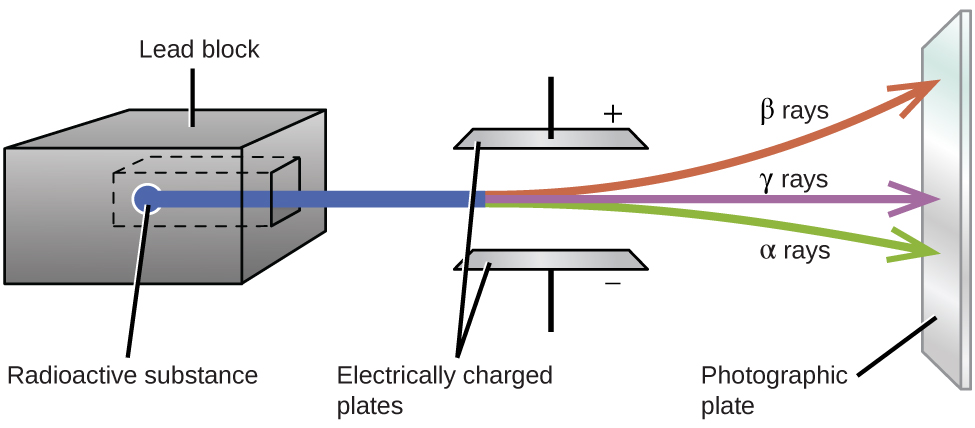
Alpha beta and gamma radiation are distinguished by their charge. In most practical laboratory sources the excited nuclear states are created in the decay of a parent radionuclide therefore a gamma decay typically accompanies other forms of decay such as alpha or beta decay. Types of radioactivity alpha beta and gamma decay chemistry libretexts. Lets discuss the properties of beta alpha and gamma one by one.
The alpha and beta radiation consist of actual matter that shoots off the atom while gamma rays are electromagnetic waves. All three kinds of radiation are potentially hazardous to living tissue but some more than others as will be explained later on. Fission is a type of radioactivity in which large nuclei spontaneously break apart into smaller nuclei.
Start studying chem 3. Alpha particle is highly active and energetic helium atom that contains two neutrons and protons. Start studying chem chapter 3.
However in order to understand the behavior of gamma rays and to compare them with alpha and beta particles a hypothetical particle called photon is introduced. After alpha or beta emission the remaining nucleus may still be in an excited energy state. Mass of alpha beta and gamma radiation.
When atoms decay they emit three types of radiation alpha beta and gamma. Alpha rays are the positively charged particles. Are atoms that have a net charge because they have gained or lost one or more electrons.
Their behavior differs from one another though all the three causes some ionization and carry some penetration power. Gamma rays have no electrical charge associated with them. Gamma rays are emitted by most radioactive sources along with alpha or beta particles.
By their charge mass and penetrating power. Learn vocabulary terms and more with flashcards games and other study tools. By releasing a gamma photon it reduces to a lower energy state.
How are alpha beta and gamma radiation distinguished. Learn vocabulary terms and more with flashcards games and other study tools. Although alpha and beta rays are composed of particles gamma rays are not composed of actual particles.
Gamma rays are emitted by unstable nuclei in their transition from a high energy state to a lower state known as gamma decay. Alpha beta and gamma radiation are distinguished by their charge and penetrating power. The major types of radioactivity include alpha particles beta particles and gamma rays.
Alpha particles are made of four nucleons. Particle with a 2 charge that is emitted by radioactive elements. Therefore they have a mass of approximately where so the mass of an alpha particle is 66410 27 kg 373 gevc 2.
 21 3 Radioactive Decay Chemistry
21 3 Radioactive Decay Chemistry
opentextbc.ca

www.scribd.com
Atoms Nuclides And Radioisotopes Canadian Nuclear Safety
www.nuclearsafety.gc.ca
 Gamma Ray An Overview Sciencedirect Topics
Gamma Ray An Overview Sciencedirect Topics
www.sciencedirect.com
 Proportional Counter Proportional Detector
Proportional Counter Proportional Detector
www.nuclear-power.net
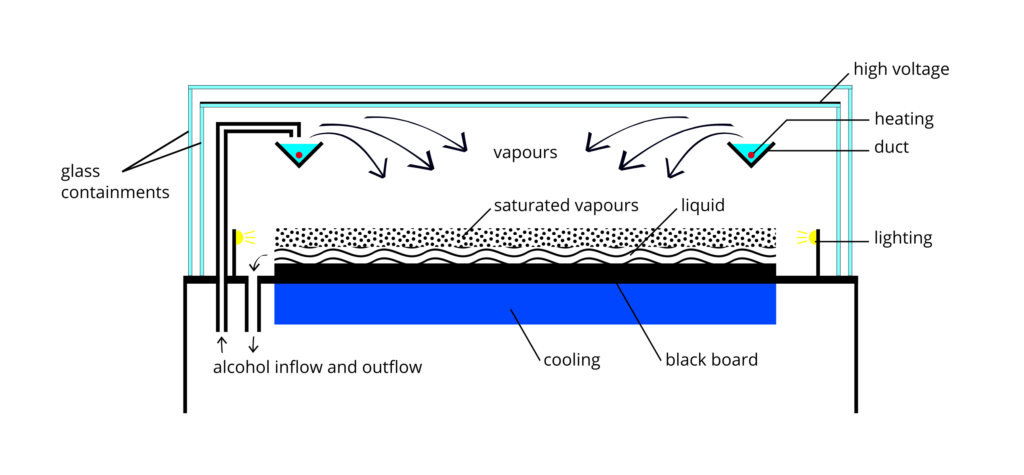 Cloud Chambers Nuledo
Cloud Chambers Nuledo
www.nuledo.com
Atoms Nuclides And Radioisotopes Canadian Nuclear Safety
www.nuclearsafety.gc.ca
 Detection Of Alpha Beta And Gamma Radiation Using Scintillation
Detection Of Alpha Beta And Gamma Radiation Using Scintillation
www.nuclear-power.net
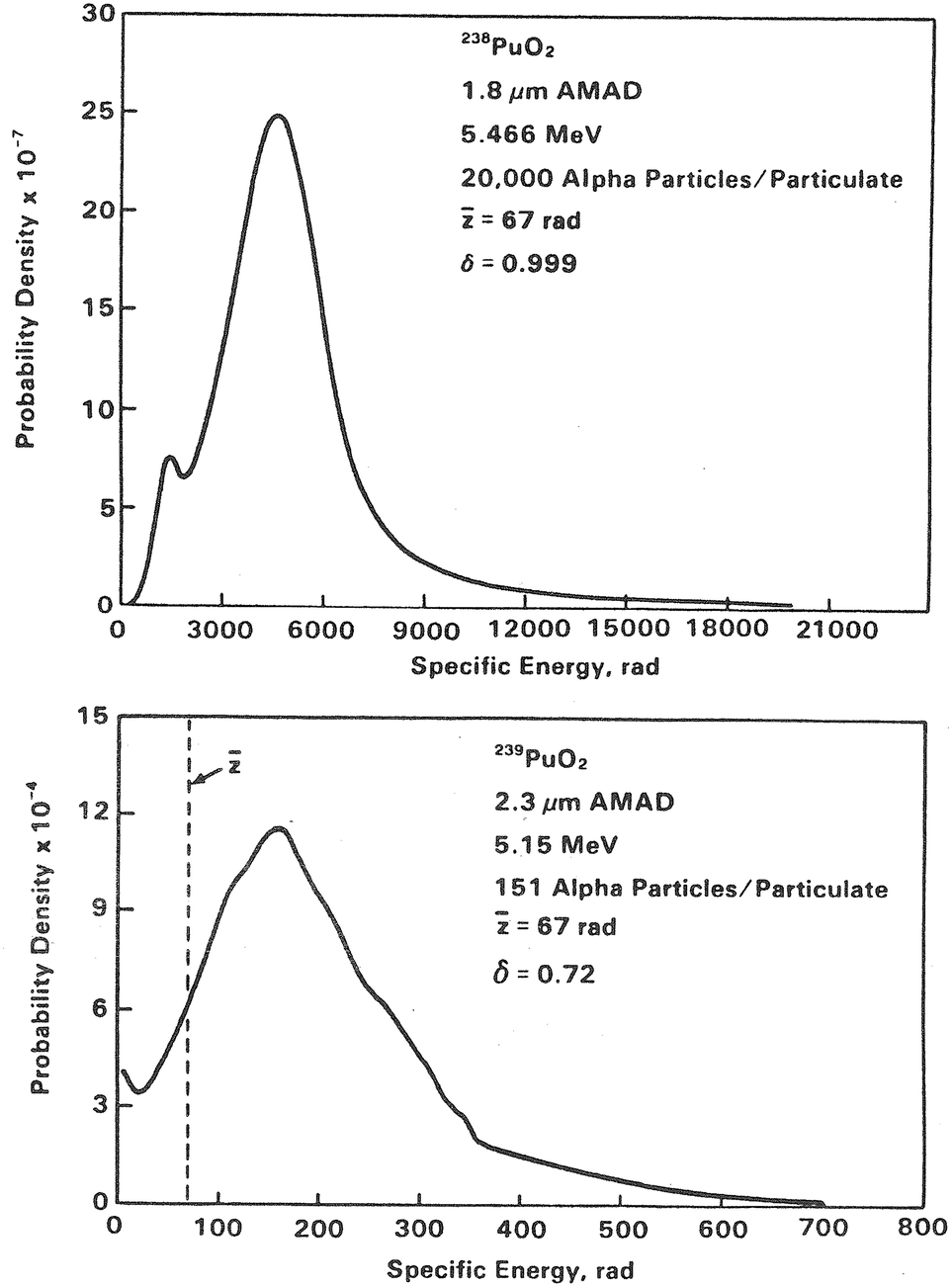 Internal Microdosimetry Of Alpha Emitting Radionuclides Springerlink
Internal Microdosimetry Of Alpha Emitting Radionuclides Springerlink
link.springer.com
0 Response to "Alpha Beta And Gamma Radiation Are Distinguished By Their Charge"
Post a Comment